BLOG > mRNA & LNP > The step-by-step guide to understand Protein Synthesis and Gene Expression
Summary of the different steps for protein synthesis and gene expression:
Step 1: Transcription: DNA to mRNA
Step 3: Translation: mRNA to Protein
Step 4: Post-Translational Modifications (PTMs)
Step 5: Visualisation of gene expression
Step 6: Regulation of gene expression
Protein synthesis is a cornerstone of molecular biology, where genetic information encoded in DNA is transcribed into messenger RNA (mRNA) and then translated into functional proteins. This intricate process is pivotal for cellular functions and underpins biotechnological applications such as recombinant protein production and therapeutic development.
In this article, we'll address how gene expression leads to protein synthesis, going through all these different steps.
Step 1: Transcription: DNA to mRNA
Transcription, the first step of protein synthesis and gene expression regulation, is initiated when RNA polymerase binds to promoter regions on DNA, assisted by transcription factors. This process involves three key steps:
- Initiation: RNA polymerase unwinds the DNA and begins synthesizing RNA at the transcription start site.
- Elongation: The enzyme progresses along the DNA template, adding ribonucleotides complementary to the DNA sequence.
- Termination: Transcription ends when specific termination signals are reached, leading to the release of the mRNA transcript. Transcription termination varies across organisms: in prokaryotes, it relies on intrinsic sequences or rho-dependent mechanisms, while in eukaryotes, transcript polyadenylation plays a key role. This process varies across organisms: in prokaryotes, it relies on intrinsic sequences or rho-dependent mechanisms, while in eukaryotes, transcript polyadenylation plays a key role.
Step 2: mRNA Processing
Before exiting the nucleus, the primary transcript (pre-mRNA) undergoes several modifications:
- 5' Cap Addition: Stabilizes mRNA and facilitates translation initiation.
- Splicing: Introns are removed, and exons are joined by the spliceosome, producing mature mRNA. Alternative splicing enables the generation of multiple protein isoforms from a single gene.
- 3' Poly(A) Tail Addition: Protects against degradation and regulates translation efficiency.
These modifications are essential for mRNA stability, efficient translation and accurate protein synthesis in the cytoplasm.
Step 3: Translation: mRNA to protein
Translation is the central mechanism of protein synthesis, converting the genetic code in mRNA into a polypeptide sequence through three phases:
- Initiation: The 40S/60S ribosome (eukaryotes) or 30S/50S ribosome (prokaryotes) assembles at the start codon, guided by sequence elements such as the Kozak sequence (eukaryotes) or Shine-Dalgarno sequence (prokaryotes).
- Elongation: The ribosome catalyzes the sequential incorporation of amino acids through interactions between mRNA codons and charged tRNA anticodons.
- Termination: A stop codon (UAA, UAG, UGA) triggers peptide release via termination factors, disassembling the ribosomal complex.
Translation efficiency and speed are modulated by cis-regulatory elements and associated proteins.
Step 4: Post-Translational Modifications (PTMs)
Various post-translational modifications are essential to ensure proper protein folding, enzymatic activity, and cellular protein synthesis regulation. These include:
- Phosphorylation: Regulates intracellular signaling pathways.
- Glycosylation: Affects stability and cell recognition of membrane and secreted proteins.
- Acetylation and Methylation: Influence transcriptional activity of histone and non-histone proteins.
- Proteolytic Cleavage: Activates inactive precursors (e.g., proenzymes, signal proteins).
In fact, these modifications will determine protein localization, interactions, and half-life.
Step 5: Visualisation of gene expression
The ability to monitor gene expression in real time is essential for understanding cellular processes. Various approaches allow visualization at different levels:
- Fluorescent and Luminescent Reporters: Reporter gene mRNAs encoding fluorescent proteins (e.g., GFP, RFP) or luminescent enzymes (e.g., luciferase) enable non-invasive tracking of gene expression dynamics.
- Fluorescence In Situ Hybridization (FISH): Detects specific mRNA molecules within cells, preserving spatial context.
- Live-Cell Imaging: Advanced techniques, such as MS2/MCP tagging systems, allow real-time tracking of mRNA localization and translation.
- Single-Molecule RNA Sequencing: Provides high-resolution quantification of transcript abundance at the single-cell level.
These methodologies provide crucial insights into gene regulation, cellular differentiation, and dynamic protein synthesis activity in living cells.
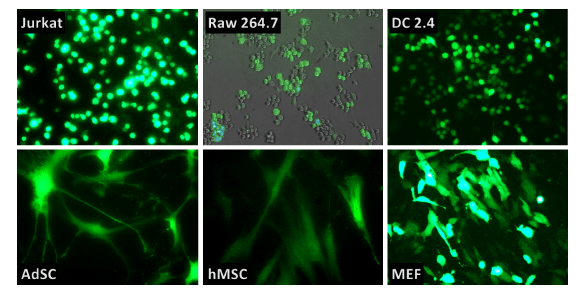
Figure 1: Jurkat T cells, Raw264.7, DC2.4, primary human Adipose Stem Cells, pri-mary human Mesenchymal Stem Cells and Mouse Embryonic Fibroblasts were transfected using RmesFect transfection reagent and mRNA-GFP from OZ Biosciences.
Step 6: Regulation of gene expression
Finally, gene expression is controlled at multiple levels:
- Transcriptional regulation: Cis-regulatory elements (promoters, enhancers) and trans-acting factors (transcription factors) define RNA polymerase accessibility. Epigenetics, via DNA methylation and histone modifications, plays a key role in gene activation or repression.
- Post-transcriptional control: RNA interference (microRNAs, siRNA) modulates mRNA stability and translation.
- Translational regulation: The activity of translation initiation factors (eIFs) and ribosomal modifications influence protein synthesis rates.
- Post-translational modifications: Protein degradation control via the ubiquitin-proteasome system or autophagy adjusts intracellular protein concentration based on cellular demands.
These regulatory mechanisms allow cells to adapt protein production to environmental cues, developmental stages, and cellular needs.
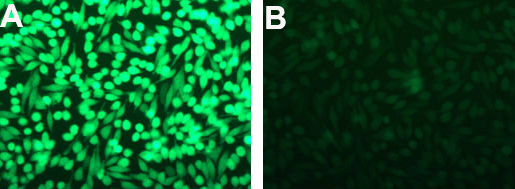
Figure 1: GFP silencing in HeLa cells. GFP-expressing HeLa cells (A) seeded in a 24-well plate were transfected with 1μL Lullaby + 5nM (33.75ng) siRNA (B) . GFP extinction was monitored 72h post-transfection by fluorescence microscopy.
Biotechnological applications of gene expression and protein synthesis
Efficient nucleic acid delivery is fundamental for studying gene function, modulating gene expression, and advancing protein synthesis research. Transfection reagents enable the introduction of DNA, siRNA, and mRNA into cells, facilitating gene overexpression, knockdown, and genome editing in both in vitro and in vivo models. In parallel, mRNA-based technologies are driving innovations in protein synthesis for applications in regenerative medicine, immunotherapy, and protein replacement therapies. Optimized mRNA synthesis and delivery enhance transcript stability, translation efficiency, and cellular uptake, ensuring robust and reproducible gene expression for research and therapeutic development.
Discover our Transfection reagents range