For Vaccination Research
Immune responses are categorized into two types: innate and adaptive (Figure 1). Cells of the innate immune system recognize and respond to pathogens in a non-specific way. The numerous types of cells involved in an immune response are dendritic cells –DC, macrophages, mast cells, eosinophils, neutrophils, B and T lymphocytes [1]. Antigen presenting cells (APCs) engulf invading pathogens, digest them and migrate to the local lymph node, where they present the antigens to naïve T cells and direct the differentiation of T helper into different effector cells (CD8+ T Cells/Th1; CD4+ T Cells/Th2).
The innate immune system provides an immediate defense against infection and is essential for the effective induction of adaptive immunity [2, 3]. Adaptive immunity in contrast, involves antigen-specific responses mediated by T cells, B cells and memory cells. T and B cells express unique T-cell receptors (TCR) and B-cell receptors (BCR) respectively, and recognize a variety of antigens. When T and B cells are activated, they lead to killer cytotoxic T cells development (cellular immunity) and antibodies production by differentiated plasma B cells (humoral immunity). The initiation of an immune response requires collaboration between innate immune cells and the adaptive immune system.
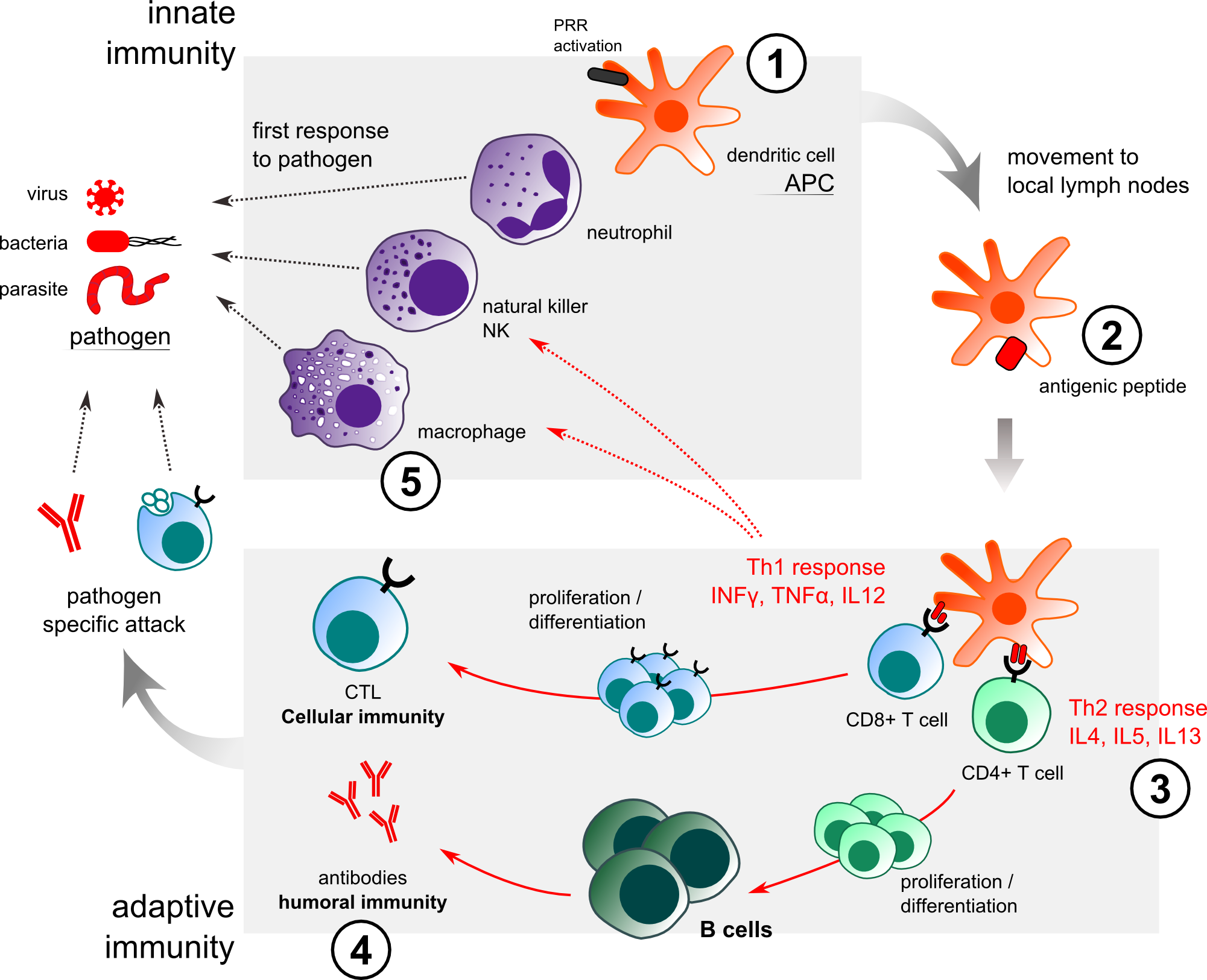
Figure 1. Innate and immune response to pathogen/adjuvant actions. VaxAlum / SqualVax / IFAVax: (1) recruitment of APC at the injection site. (2) enhancement of antigen uptake. (3) enhancement of Th2 response. (4) enhancement of humoral immune response. (5) AlumVax: improvement of NALP3/inflammasome in macrophages.
ROLE OF ADJUVANTS IN VACCINES & ANTIBODY PRODUCTION
Three main types of vaccine are generally employed: i) live-attenuated vaccines, ii) inactivated vaccines that are heat- or chemically- killed microorganisms and iii) sub-unit vaccines that are made from components of the pathogen such as proteins, peptides or genetic materials. Immunization or vaccines induce pathogen-specific adaptive immunity by generating memory cells against a specific pathogen [2]. Any vaccine consists of a specific antigenic part of the pathogen, that will instigate a T and B lymphocytes response with production of memory cells. Following administration, the antigen is taken up and presented by APCs as peptide/MHC protein class I or II complexes which binds the T cell Receptors (TcR) of T Helper cells CD8+ (Th1) and CD4+ (Th2) respectively. Under the priming by APC through secretion of cytokines, T helper cells differentiate into two major sub-types: Th1 and Th2 cells. IL-2 and IL-12 promote Th1 differentiation leading to INF-g, TNF-a and IgG2a release and proliferation of cytotoxic CD8+ T cells. This is the “cellular response” that fights intracellular pathogens. IL-1b and IL-18 induce Th2 differentiation leading to secretion of IL-4, -5, -6, -10 and -13 and proliferation of antibody secreting B-cell: this is the “humoral response” that protects the organism against extracellular pathogens. The immune response induced by antigenic or genetic immunization can thus generally be distinguished by these two different responses: Th1 vs Th2.
Successful vaccines for certain pathogens will likely require enhanced immune responses including Th1-cellular-mediated immunity or a more robust Th2-humoral response.
For antibody production, a strong Th2 response will be preferred. The quality of the vaccine-induced immune response or level of antibody production will depend on several factors including, the route, number and timing of administrations, the nature of the antigen and the quality of antigen presentation. This whole process is facilitated by the adjuvants. Indeed, adjuvants allow to overcome the poorly immunogenic properties of most protein, peptide and DNA vaccines (lacking natural immune triggers) or the induction of inappropriate immune responses.
Thus adjuvants can be used to (1) enhance the immune response, (2) orient the immune response through modulation of the Th1/Th2 balance and (3) reduce the amount of antigen needed and the number of injections required to induce protection (antigen-dose sparing).
HOW ADJUVANTS WORK?
Adjuvants are generally classified into two groups: vehicles and immunostimulants with some components sharing both properties. Vehicles are usually of particulate nature (mineral salts, e.g. aluminum hydroxide, emulsions, liposomes). They afford the display of selected antigens in multiple copies, mimicking natural display by microorganisms and are used to deposit the antigen at the site of administration or to increase its delivery into APCs (micro- and nanoparticles) [4]. In contrast, the immunostimulants (TLR agonists, saponins, cytokines…) directly target and activate cells of the immune system and increase the immune response to antigens (Figure 2). Within this group of adjuvants, those targeting the pathogen recognition receptors (PRRs) of the innate immune system have received considerable attention over the last decade.
Principal properties of an adjuvant:
|
Enhance speed, strength, and duration of the immune response.
|
Affords broadening of antibody cross-reactivity.
|
Select, or modulate cell-mediated virus humoral immunity:
|
Decrease the amount of antigens needed for protection (antigen dose sparing).
|
Increase immune response to vaccines in immunologically immature, immunosuppressed or senescent individuals.
|
Increase the immunogenicity of weakly immunogenic antigens.
|
Modulate antibody isotype, subclass, quantity and specificity.
|
PRRs allow innate immune cells to recognize microbial or infectious components, known as pathogen associated molecular patterns (PAMPs) and components released from injured or dying cells, known as damage associated molecular patterns (DAMPs). These PAMPs and DAMPs binding to PRRs, induce inflammatory response that will in turn enhance the adaptive immune response [5]. There are four classes of PRRs: Toll-like receptors (TLRs), Nod-like receptors (NLRs), RIG-1 like receptors (RLRs) and C-type lectin Receptors (CLRs). Nearly all members of the PRR family are considered to be receptors for adjuvants [6-9]. A third group of adjuvants formed by the combination of these immunostimulants with particulate delivery vehicles and referred to as “adjuvant systems” or “adjuvant combinations” is emerging as a group comprising some of the most potent adjuvants. This group comprises the combination of TLR agonists with Alum, emulsions or liposomes [10].
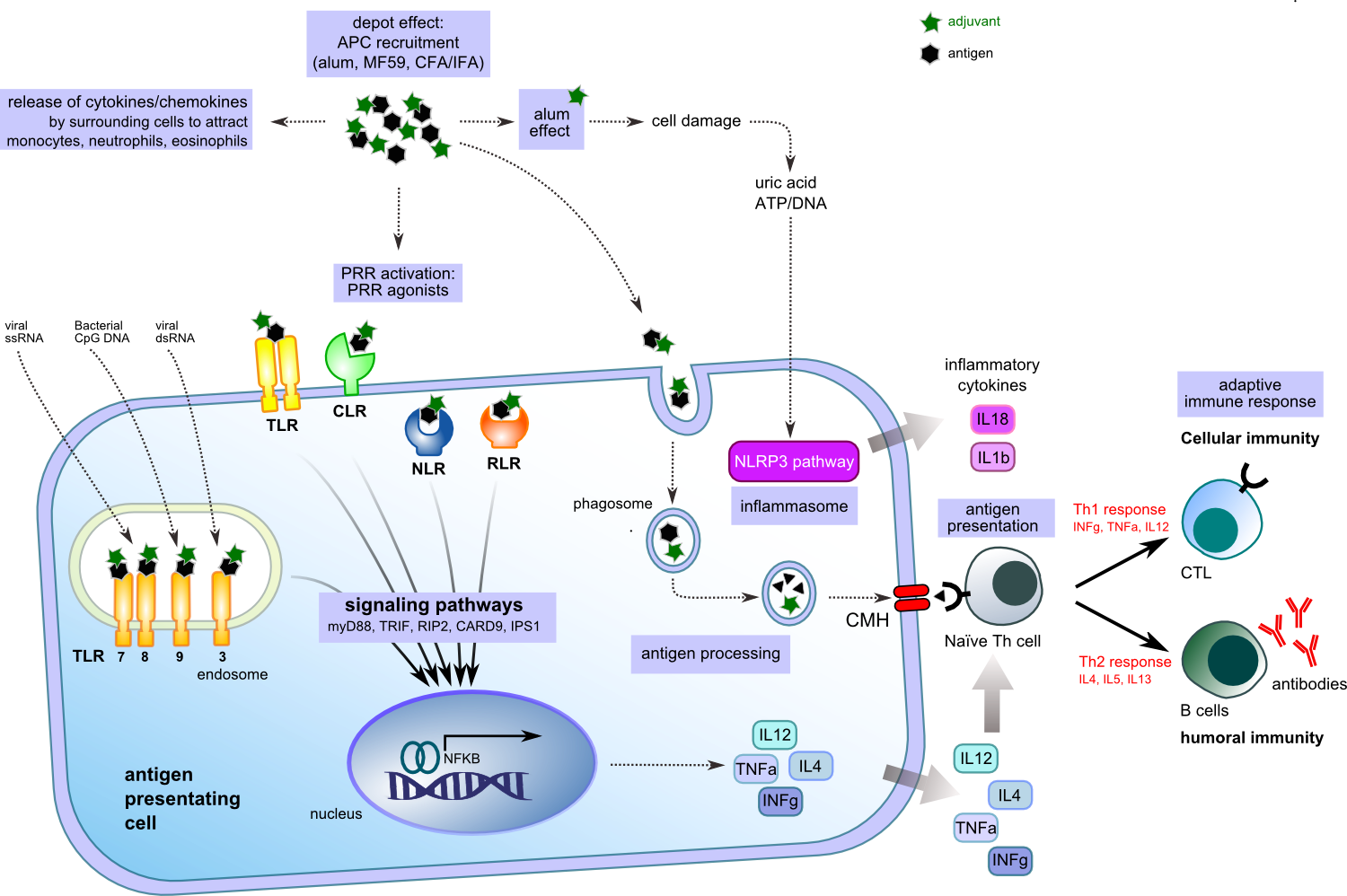
Figure 2. Adjuvanted antigen activation of APC.
Freund's Adjuvants
Incomplete Freund’s Adjuvant (IFA) = strong Th2 immune response + low Th1 response
Complete Freund’s Adjuvant (CFA) = Strong Th1 immune response
IFAVax Incomplete Freund's Adjuvant (IFA) and CFAVax Complete Freund’s Adjuvant (CFA) are water-in-oil emulsions consisting in mixtures of mineral oil and an emulsifier in a ratio of 85% v/v oil and 15% v/v emulsifier. Contrary to the Complete Freund’s Adjuvant (CFA), IFA does not contain heat-killed mycobacteria (Mycobacterum tuberculosis). IFA and CFA have been used extensively in experimental immunology and IFA has been used for decades in practical veterinary vaccination. Importantly, Freund’s adjuvants are not pre-formed emulsions and thus they must be mixed with an equal volume of aqueous solution of antigens and subsequently emulsified prior to use. Even if the mechanisms of action of oil emulsions are still poorly understood, some evidences suggest a partial requirement for NOD2. Moreover, these emulsions are prone to cause cellular damage upon injection and thus, endogenous signals released during necrotic cell death may also contribute to their adjuvant activity. Immune response directed by CFA is dramatically enhanced by the presence of the mycobacterial component that attracts macrophages and immune cells at the site of injection. CFA induces principally a Th1 response and can cause granulomas and an intense inflammatory reaction at the inoculation site. Indeed, the use of CFA should be used responsibly and with care in order to avoid or minimize the adverse effects of excessive inflammation. As opposed to CFA that induces principally a Th1 response, IFA that lacks mycobacterial components primes a Th2 response [11]. For most applications, CFA is usually only necessary for the initial immunization, while IFA is the adjuvant of choice for subsequent immunizations. IFAVax and CFAVax adjuvants are designed to provide continuous release of antigens necessary for stimulating strong persistent immune response.
Aluminium Adjuvant (for Th2 immune response)
Alum is the most common adjuvant used in approved prophylactic vaccines because of its excellent safety profile and ability to enhance protective humoral immune response (Th2). It consists of precipitates of aluminum phosphate and / or aluminum hydroxide to which antigens are adsorbed through hydrophobic and electrostatic interactions or entrapped. AlumVax hydroxide is positively charged at a physiological pH of 7.4 and binds acidic proteins. AlumVax phosphate on the other hand is negatively charged and therefore binds basic proteins. The association of antigen with Alum favors a high local antigen concentration and an improved uptake by APC. Thus early observations described that Alum enhances the response to antigens by extending the time during which the antigen is available. Furthermore, it is now admitted that Alum, like many other adjuvants, acts by direct activation of the immune cells. In mice, Alum induces a profoundly polarized Th2 immune response which is characterized by the production of IL-4 and IL-5 and the strong induction of antibodies production as immunoglobulin IgE and IgG1 [12-13].
Consequently, Alum is very effective against pathogens that require Th2 humoral-mediated immunity. In parallel, Alum alone can activate the NLRP3 inflammasome to produce mature IL-1 creating a favorable environment for the immune response [14]. Nonetheless; Alum fails to induce Th1 responses associated with the induction of INF-g and cytotoxic T lymphocytes which are required to clear the body of intracellular infections.
Different aluminium salts are contained in numerous licensed vaccines (see table below) and their safety profiles have been well established through the use of billions of doses of aluminum-containing vaccines administrated to individuals from infants to elderly [15].
Aluminium Salts
|
Vaccines
|
Al(OH)3 – aluminium hydroxide
AlPO4 – aluminium phosphate
|
Pertussis, Diphtheria
Tetanus, HBV - HAV
|
(Al)2PO4SO4OH – aluminium
|
HPV
|
HBV, hepatitis B virus; HAV, hepatitis A virus; HPV, human papillomavirus |
|
Squalene emulsion (Strong immune response – balance Th1/Th2 response)
SqualVax is an oil-in-water emulsion made of squalene droplets in a continuous aqueous phase. Squalene droplets are stabilized by the addition of two non-ionic surfactants that are widely used as emulsifiers in food, cosmetics and pharmaceuticals [16]. It is fully biodegradable, which is an important advantage over alternative oils used in emulsion adjuvants, like Freund’s adjuvant that contains mineral oil (paraffin oil) and thus has long term persistence in the organisms.
Squalene emulsion induces local stimulation and recruitment of DCs and granulocytes, differentiation of monocytes into DCs [17] and increased uptake of antigen by APCs [18]. The emulsion acts more specifically on macrophages present at the site of injection. A local increase in chemokines release also influences the recruitment of immune cells from the blood to the site of vaccination, creating an amplification loop. This type of formulation enhances differentiation of monocytes towards a mature phenotype, thereby promoting migration of antigen-loaded cells to the draining lymph node. Compared to aluminium salts, a stronger immune response is elicited (e.g higher antibody and T-cell response) with a mixed and more balanced Th1/Th2 cell phenotype [17]. Squalene emulsions are present in licensed seasonal and pandemic influenza vaccines. They enhance immune response in a challenging population such as elderly and can facilitate immune response against specific drift variants of the seasonal influenza virus not included in the vaccine [19].
NEW! Now we also offer SqualVax Vegetal, a phytosqualene-based oil-in-water emulsion. Similar to the SqualVax vaccine adjuvant, except that squalene sourced from animal origins has been advantageously replaced with phytosqualene (plant-derived) extracted from first choice European olive oil.
SqualVax formulation is similar to the MF59® (a registered trademark of Novartis). MF59 is used for comparative purposes only. SqualVax™ is not made by, affiliated with, sponsored by or endorsed by Novartis. Novartis has not evaluated or approved the representations contained herein).
BIBLIOGRAPHIC REFERENCES
- 1. van Kooyk Y., et al., Curr Opin Immunol, 2004. 16(4): p. 488-93.
- 2. Akira S., Philos Trans R Soc Lond B Biol Sci, 2011. 366(1579): p. 2748-55.
- 3. Iwasaki A. and R. Medzhitov, Science, 2010. 327(5963): p. 291-5.
- 4. Cox J.C. and A.R. Coulter, Vaccine, 1997. 15(3): p. 248-56.
- 5. Medzhitov R. and C. Janeway Jr., N Engl J Med, 2000. 343(5): p. 338-44.
- 6. Elinav E., et al., Immunity, 2011. 34(5): p. 665-79.
- 7. Kawai T. and S. Akira, Immunity, 2011. 34(5): p. 637-50.
- 8. Loo Y.M. and M. Gale Jr., Immunity, 2011. 34(5): p. 680-92.
- 9. Osorio F. and C. Reis e Sousa, Immunity, 2011. 34(5): p. 651-64.
- 10. Reed S.G et al., Nat Med, 2013. 19(12): p. 1597-608.
- 11. Lindblad E.B., et al., Infect Immun, 1997. 65(2): p. 623-9.
- 12. Marrack P., A.S. McKee, and M.W. Munks, Nat Rev Immunol, 2009. 9(4): p. 287-93.
- 13. Aimanianda V., et al., Trends Pharmacol Sci, 2009. 30(6): p. 287-95.
- 14. Li H.S. Nookala, and F. Re, J Immunol, 2007. 178(8): p. 5271-6.
- 15. Singh M., et al., Vaccine, 2006. 24(10): p. 1680-6.
- 16. O'Hagan D.T., Expert Rev Vaccines, 2007. 6(5): p. 699-710.
- 17. Seubert A., et al., J Immunol, 2008. 180(8): p. 5402-12.
- 18. Dupuis M., et al., Cell Immunol, 1998. 186(1): p. 18-27.
- 19. Podda A., Vaccine, 2001. 19(17-19): p. 2673-80.
Discover our other applications

Virus concentration
Mag4C magnetic nanoparticles capture viruses in culture medium by electrostatic and hydrophobic interactions with 80-99 % efficiency. Once captured onto magnetic beads, viruses can be...

Stem workflow
Stem cells are widely investigated due to their regenerative potential. In many cases, it would prove useful to enhance or reduce some of their features through gene delivery strategies...

3D Transfection
Three-dimensional (3D) matrices, such as 3D-scaffolds and 3D-hydrogels, work as mechanical platforms for cell attachment and growth.