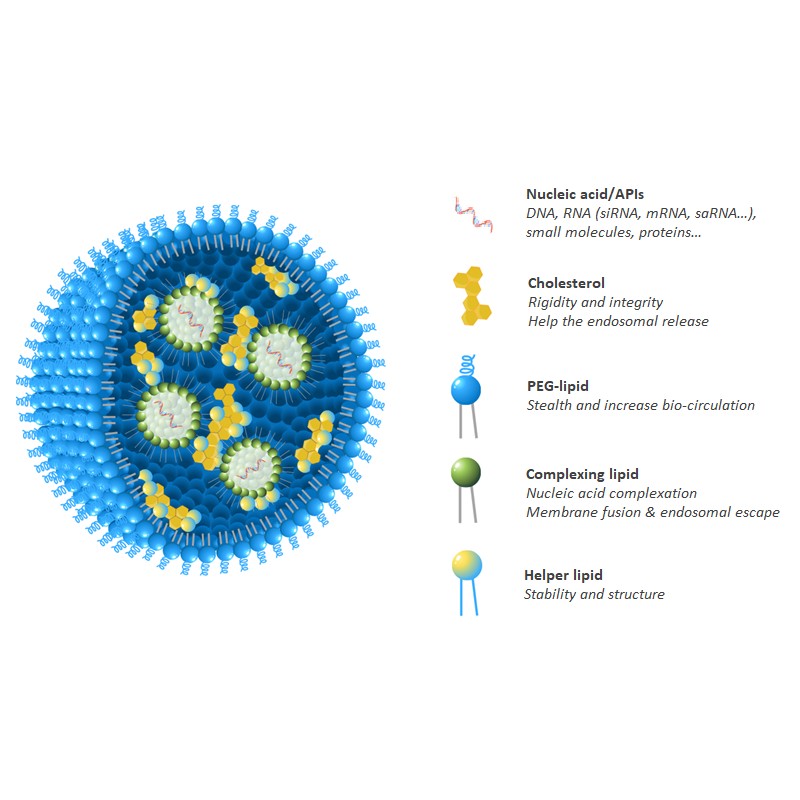
Highly Potent Non-Viral Drug Delivery Nanosystems
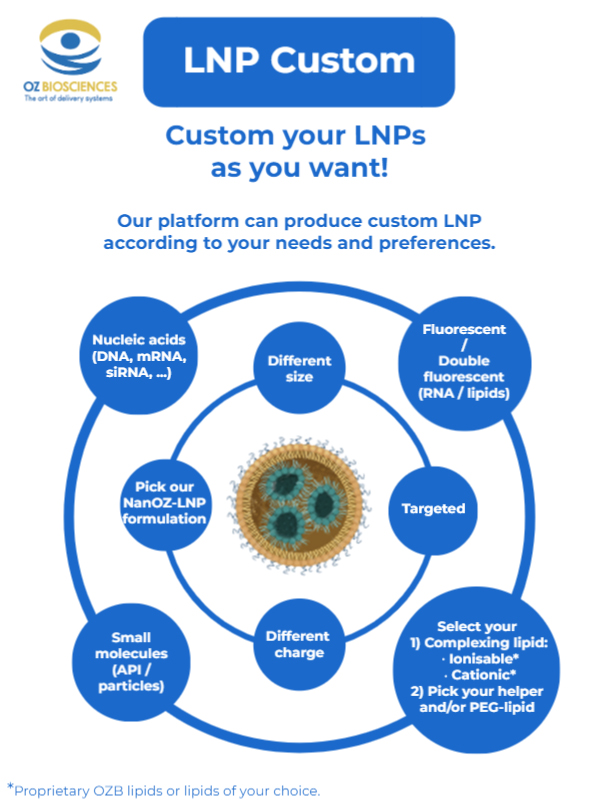
OZ Biosciences LNP Custom Service
OZ Biosciences has developed a Microfluidics Platform for the reproducible development of safe & potent drug delivery vehicles for pharmaceutical applications.
We can support every stage of your mRNA-LNP production, from mRNA synthesis to LNP formulation development, manufacturing and fill & finish.
For any of RNA, DNA or APIs encapsulation, you can provide us with your molecule of interest and we will formulate it into LNPs.
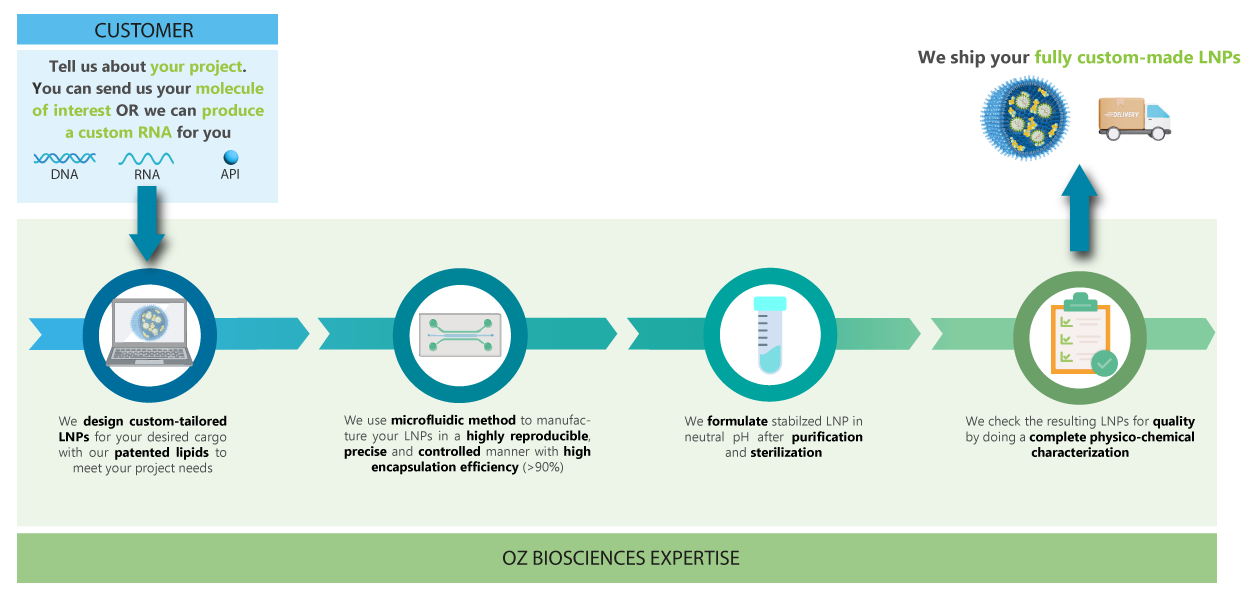
Discover our Custom LNP service worklow.
Our team is dedicated in providing you with high quality LNP that will meet your project needs. You can provide your own molecule of interest to encapsulate or we can make a custom mRNA for you.
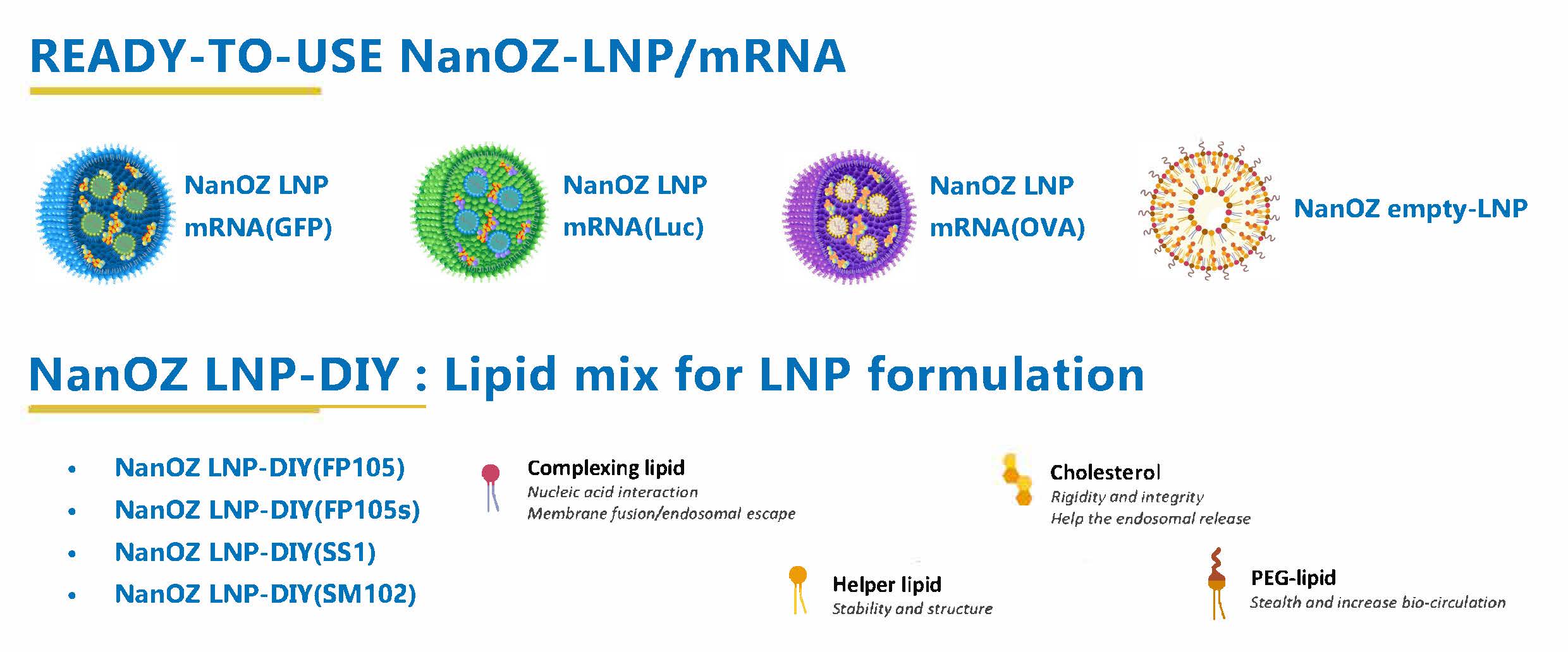
Aside from our custom service, we also offer ready-to-use NanOZ-LNP/mRNA products and NanOZ LNP-DIY: Lipid mix for LNP formulation for your research studies:
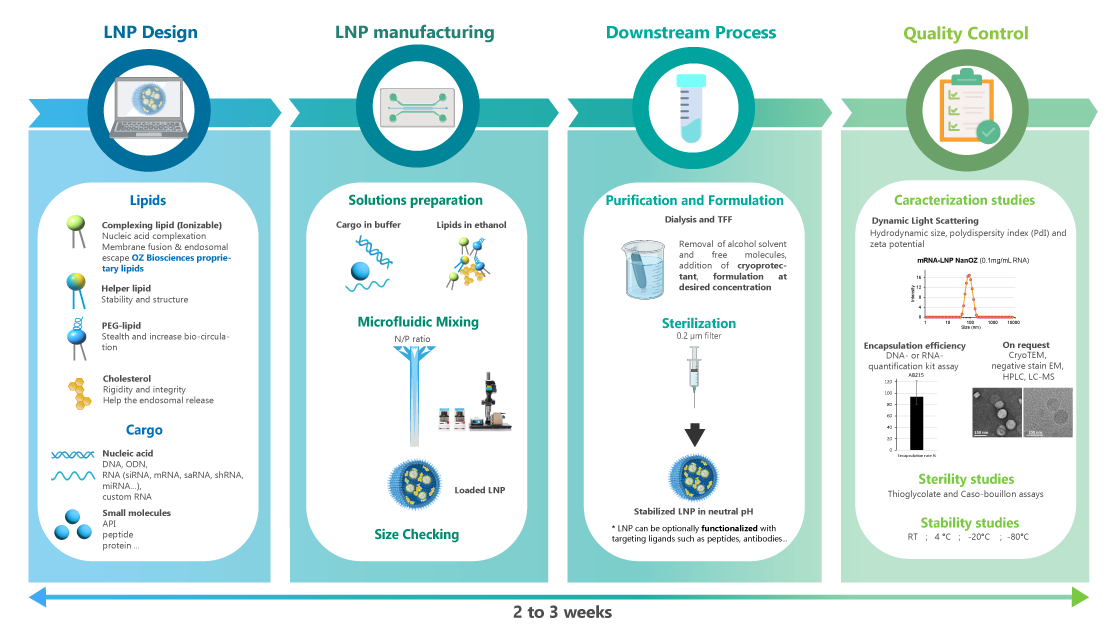
Physico-chemical characterization of LNPs
NanOZ-LNPs are formulated through pressure-driven controlled flow microfluidics systems. Once collected and purified, NanOZ-LNPs physico-chemical properties are fully characterized in terms of size distribution, charge surface, structure integrity, encapsulation efficiency and stability.
The NanOZ-LNP/mRNA are monodisperse nanoparticles of spherical morphology with size usually ranging between 80-150nm and PDI<0.2. Once encapsulated, the integrity of the mRNA is verified by gel electrophoresis.
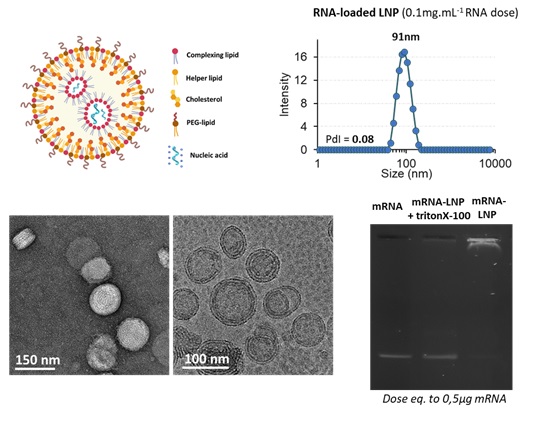
Fig. 1: Physico-chemical characterizations of NanOZ-LNP/mRNA: Size distribution and Polydispersity Index measurements by DLS, morphology and monodispersity observed by colour negative SEM and CryoTEM micrographies and mRNA integrity after formulation inside LNP monitored by agarose gel electrophoresis at mRNA dose equals to 0.5µg. Data Source: The results obtained by OZB, Marseille.
NanOZ-LNP in vivo data
The biodistribution and transfection efficiency of NanOZ-LNP loaded with Firefly Luciferase mRNA (mRNA-Luc) was evaluated after i.p. injection of 10µg mRNA formulated in LNP in nude mice with two formulations based on proprietary ionizable lipids compared with a LNP formulation based on DOTAP cationic lipid. Bioluminescence intensity was monitored by IVIS instrument over 25h.
As observed in Figure 2, a single intra-peritoneal (i.p.) injection of Luc-LNPs enables high bioluminescence signal and luciferase expression into surrounded organs for at least 25h (Figure 4). The highest transfection efficiency was observed for the NanOZ-LNP1 formulation.
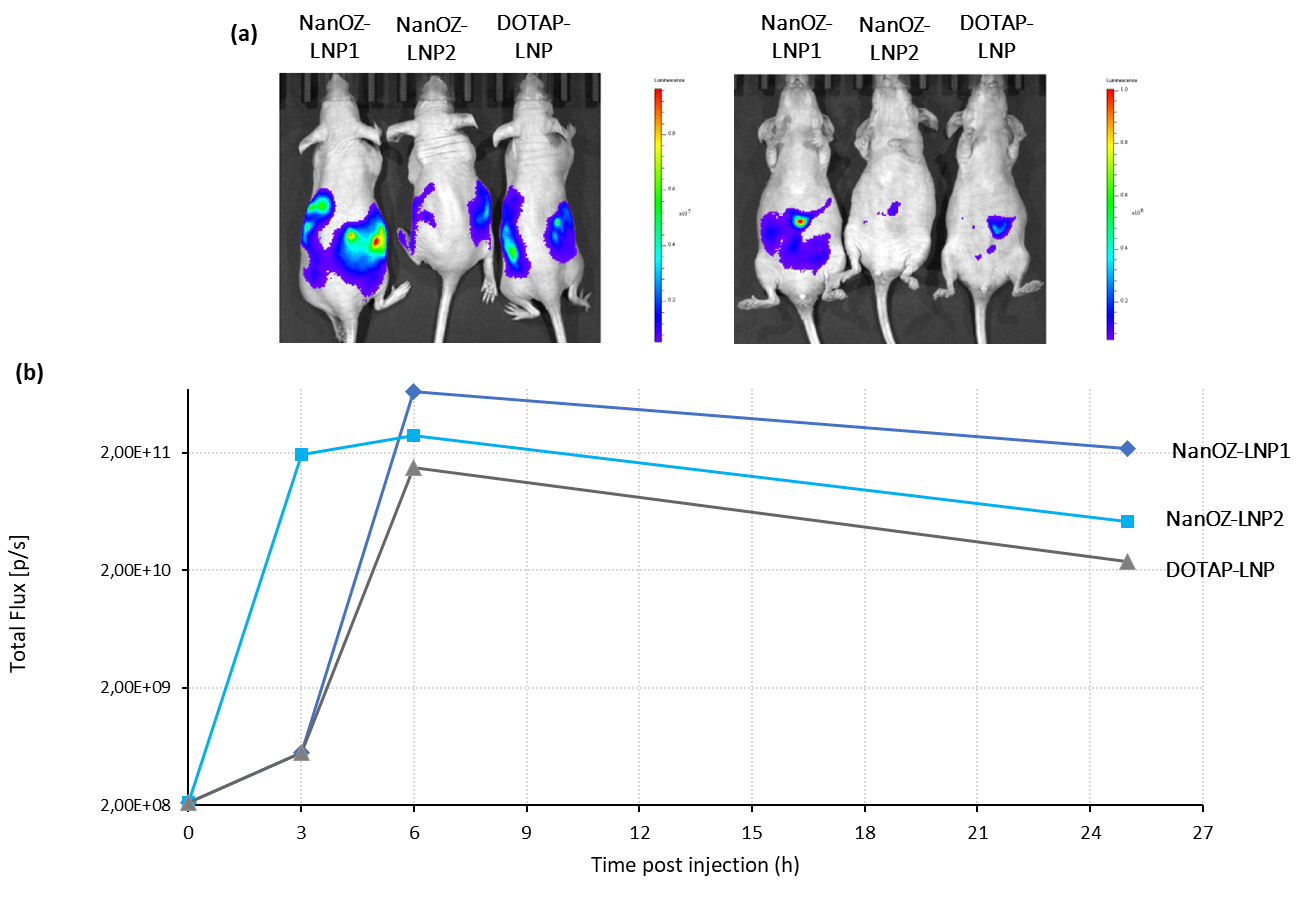
Fig. 2: IVIS images 6h after i.p. administration of 3 mRNA-LNPs formulation: NanOZ-LNP1, NanOZ-LNP2 and DOTAP-LNP in nude mice. Kinetics of bioluminescence signal over 25h after i.p. administration of 3 mRNA-LNPs formulation: NanOZ-LNP1, NanOZ-LNP2 and DOTAP-LNP in nude mice (dose equivalent to 10µg mRNA). Data Source: The results were acheived in collaboration with the TRACE PDX Platform, KU Leuven.
NanOZ-LNP in vivo immunization results
The comparison between mRNA-based vaccines versus subunit vaccine for immune efficacy were evaluated in C57BL6J mice using the ovalbumin model. Prime boost (D0-14) immunizations were evaluated with OVA antigen delivered by mRNA-based LNPs, where two different formulations were tested corresponding to LNP1 and LNP2, or by recombinant protein (alone or adjuvanted). To characterize the immuno-phenotyping of the mice the sera were collected at D0, D14, D21 and D28 and splenocytes were collected at D28 and analysed by ELISA, Elispot and Multiplex. The mice were immunized by s.c. route with 10µg OVA subunit (SU) or 10µg mRNA doses (100µL per injection).
RNA vaccine LNP formulations are efficient at priming a strong Th1 humoral response equivalent to the best TH1 adjuvants such as QS-21 upon sub-cutaneous prime boost injection (D0-14) in C57BL6J mice. In addition, no significative signal was observed when the OVA antigen is directly administrated. It is noteworthy, the necessity of two injections (boost at D14) to obtain a long-lasting immune response.
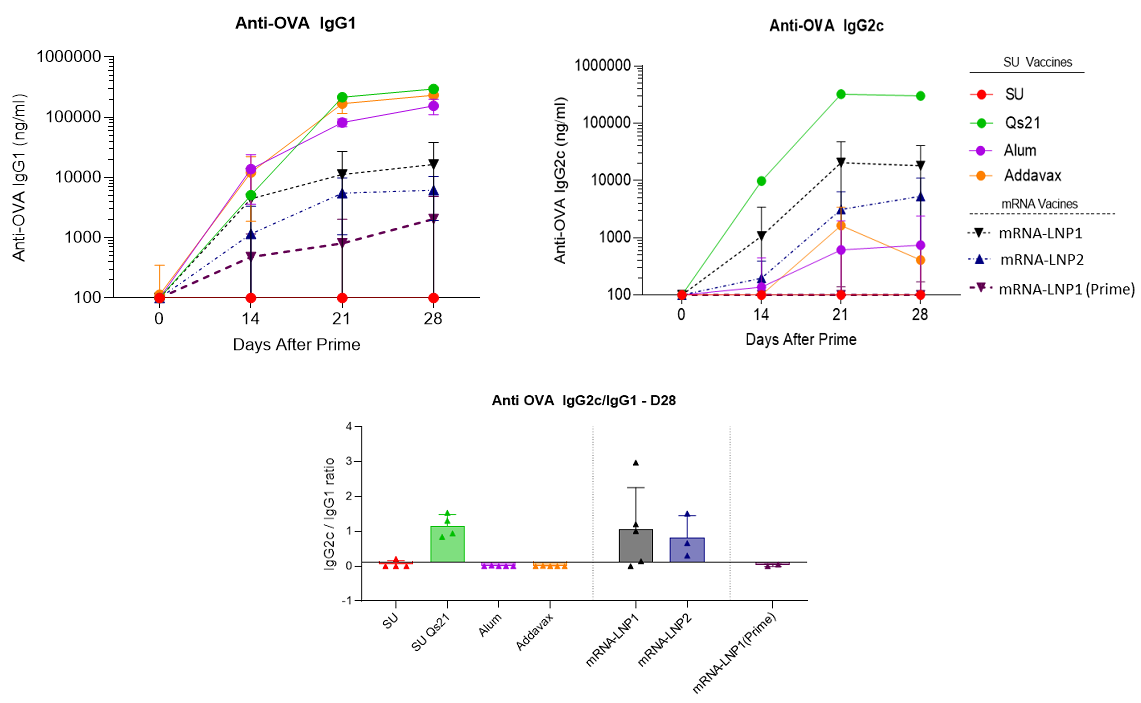
Fig. 3: Anti-OVA IgG production and Th1 humoral biomarker to OVA subunit vaccine (10µg) in absence or presence of vaccines adjuvants (QS21 (5µg), Alum (Aluminum Hydroxide gel)1X or addaVax 1X) and to mRNA(OVA)-LNPs (mRNA dose equals to 10µg) upon sub-cutaneous prime boost injection (D0-14) in C57BL6J mice. Data Source: The results were acheived by OZ Biosciences in collaboration with Dr. Antoine Tanne, SaponiQx, Lexington, MA, USA
NanOZ-LNP in vitro evaluation
Data show that LNP carrying mRNA can be used for transfecting cells in vitro but with less efficiency than classic transfection reagents such as RmesFect: at least 10 times more material would be needed using LNP. Depending on the cell line (such as JurKat T cells cultivated in suspension) LNP could be an alternative to transfection reagents for genetic modification with a very high efficiency; however, the fluorescence intensity that could account for the number of delivered molecule is still below.
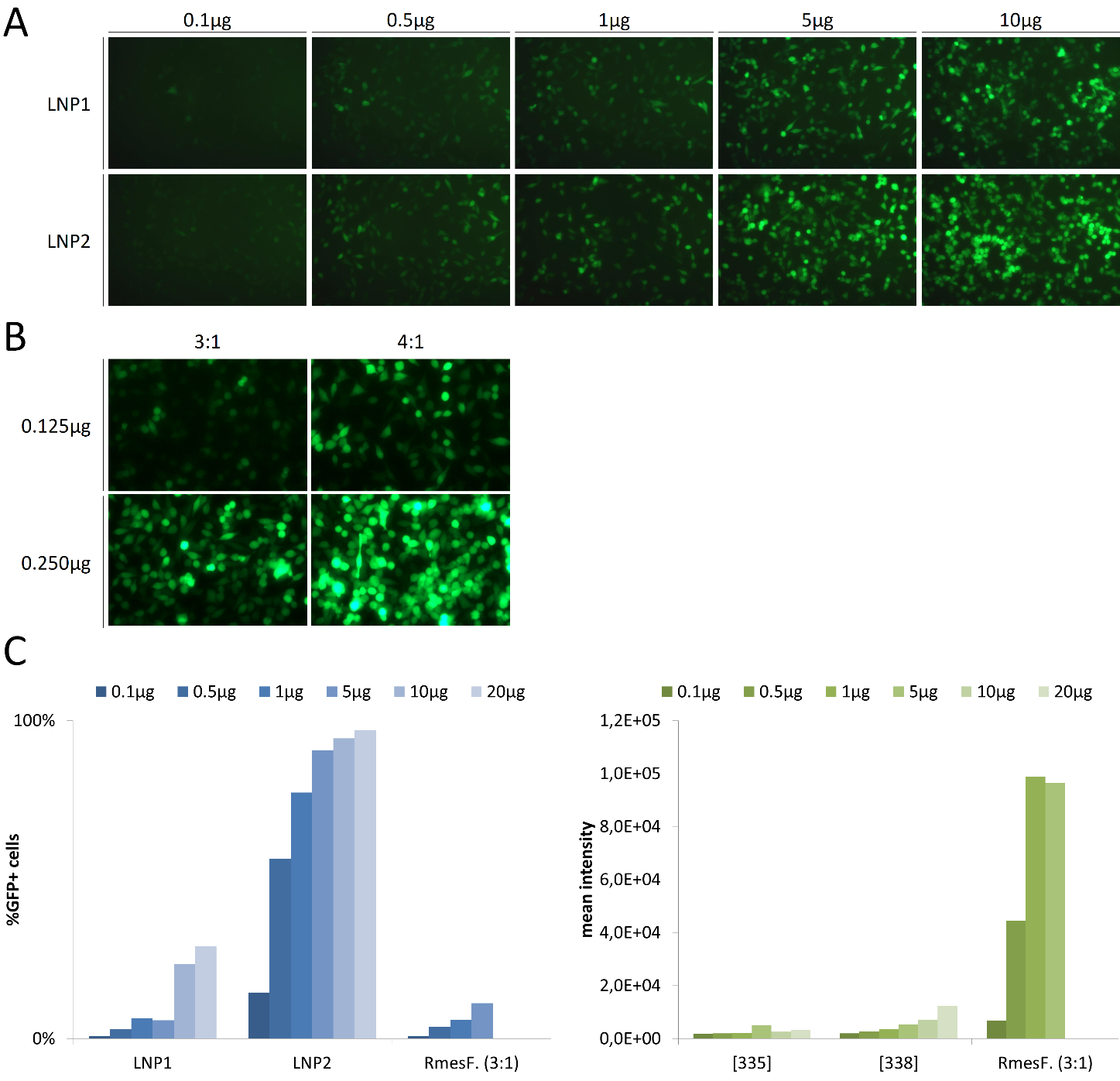
Figure 4: In vitro transfection using LNP and Lipoplexes. (A) HeLa cells cultivated in 24-well plates were transfected with ranging doses of mRNA (from 0.1 µg to 10µg) encoding for GFP encapsulated with LNP1 and LNP2. (B) HeLa cells were transfected with 0.125 and 0.25µg mRNA encoding for GFP complexed to RmesFect transfection reagent using two ratios, 3:1 and 4:1 (respectively 3µL and 4µL per µg mRNA). GFP expression in (A) and (B) was monitored by fluorescence microscopy 48H after transfection. (C) Jurkat T cells were transfected using ranging doses of mRNA encapsulated with LNP1 and LNP2 or complexed with RmesFect (ratio 3:1). 48H after transfection, percentage of transfected cells (left graph) and fluorescence intensity (right graph) were determined by flow cytometry.