BLOG > Publications & Citations > TREK-1 in regulating adipocyte differentiation and lipid accumulation
Authors: Kim, A., Jung, S., Kim, Y. et al.
Source: Cell Death Dis 16, 164 (2025).
We are thrilled to share insights from a genuine study entitled "Novel function of TREK-1 in regulating adipocyte differentiation and lipid accumulation" published in Nature Cell Death & Disease by Ajung Kim, Seoyeong Jung, Yongeun Kim et al:
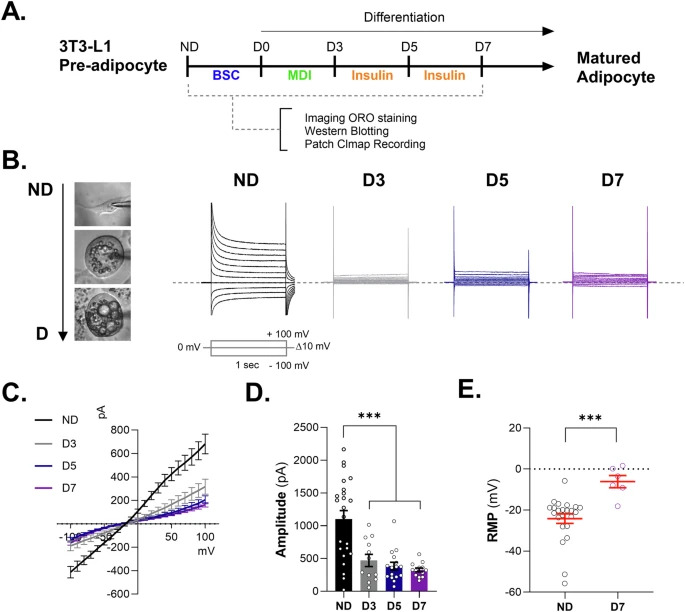
"K2P (two-pore domain potassium) channels, a diversified class of K+-selective ion channels, have been found to affect a wide range of physiological processes in the body. Despite their established significance in regulating proliferation and differentiation in multiple cell types, K2P channels’ specific role in adipogenic differentiation (adipogenesis) remains poorly understood. In this study, we investigated the engagement of K2P channels, specifically KCNK2 (also known as TREK-1), in adipogenesis using primary cultured adipocytes and TREK-1 knockout (KO) mice. Our findings showed that TREK-1 expression in adipocytes decreases substantially during adipogenesis. This typically causes an increased Ca2+ influx and alters the electrical potential of the cell membrane in 3T3-L1 cell lines. Furthermore, we observed an increase in differentiation and lipid accumulation in both 3T3-L1 cell lines and primary cultured adipocytes when the TREK-1 activity was blocked with Spadin, the specific inhibitors, and TREK-1 shRNA. Finally, our findings revealed that mice lacking TREK-1 gained more fat mass and had worse glucose tolerance when fed a high-fat diet (HFD) compared to the wild-type controls. The findings demonstrate that increase of the membrane potential at adipocytes through the downregulation of TREK-1 can influence the progression of adipogenesis."
Congratulations to all authors for this great article.
Our CombiMag was used to improve the efficiency of cell transfection, by L2000 of shRNA in mouse inguinal white adipose tissue stromal vascular cells (iWAT SVC).
Read the article See our CombiMag