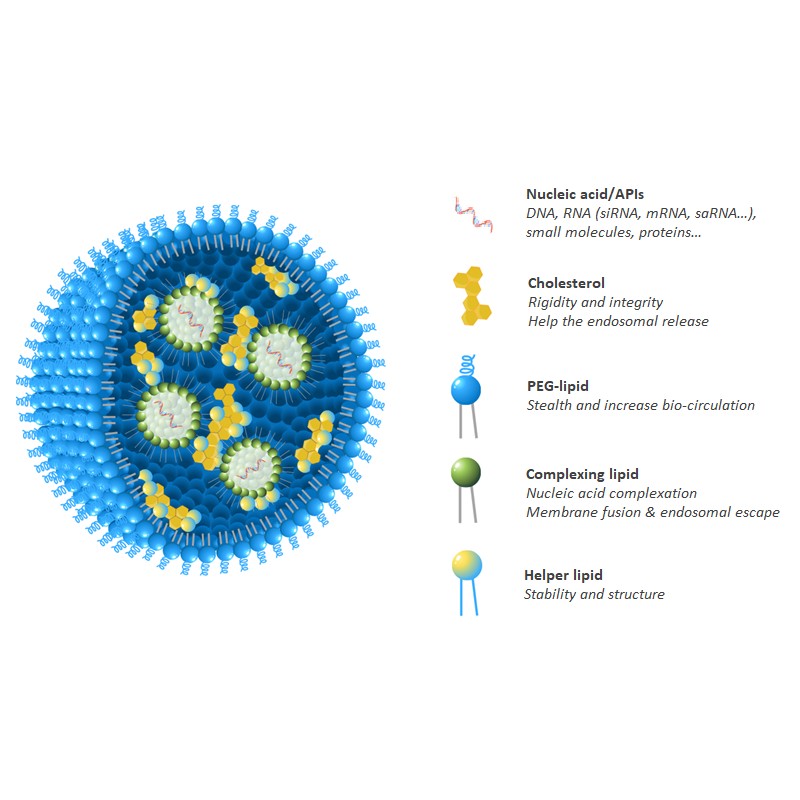
A New Transfection Reagent, not Just Another One
After the development of Lipofection (lipid-based transfection method) and Magnetofection (magnetic nanoparticles-based transfection method), OZ Biosciences revolutionizes Polyfection with the design and synthesis of a novel patented Cationic Hydroxylated Amphiphilic Multi-block Polymer (CHAMP) which is biocompatible, cleavable, ph responsive and bi-functional.
We created a totally new transfection agent based on the CHAMP technology to mark the separation from what is usually being done with classic transfection methods. This novel bi-functional copolymer is biocompatible, ionizable and pH sensitive. Formed by three moieties, it combines and introduces three synergistic notions:
- The concept of “passing through the membranes barriers” due to its charge, pH-sensitive and hydrophobic properties.
- The idea of “stealth transfection” where DNA is protected, masked and supported all the way to its nuclear uptake.
- The concept of biocompatibility due to biodegradable and cleavable moieties
This polymer-based transfection technology is an optimized delivery system that allows high efficiency with low cellular stress thanks to improved delivery mechanisms.
HISTORY
Two main types of delivery vehicles are routinely used for genetic modification of cells: viral and non-viral vectors.
Transport systems have to overcome a series of extracellular and intracellular barriers until the DNA delivery into the cell nucleus. Viruses have evolved in order to bypass each of these checkpoints but despite their efficiency they have to deal with important issues such as immunogenicity, cytotoxicity, safety and target-cell specificity that limit their use.
 Inspired by the strategy of some viruses to gain entry into mammalian cells, researchers tried to build synthetic viruses or virus-like particles in order to efficiently and safely transport genetic material to the cell nucleus; the main focus being to mimic viral vectors in terms of performance without encountering their principal pitfalls[i]. The goal was to replicate with the synthetic molecular complexes all the steps used by the viruses to infect mammalian cells.
Inspired by the strategy of some viruses to gain entry into mammalian cells, researchers tried to build synthetic viruses or virus-like particles in order to efficiently and safely transport genetic material to the cell nucleus; the main focus being to mimic viral vectors in terms of performance without encountering their principal pitfalls[i]. The goal was to replicate with the synthetic molecular complexes all the steps used by the viruses to infect mammalian cells.
Non-viral vectors have thus gained increasing attention since several decades as they do not contain any pathogenic proteins and are therefore more likely to be safe[ii]. However, synthetic carriers were generally unsatisfactory because they lacked one or several of the necessary functions needed for optimal performance.
Synthetic vectors are materials that electrostatically bind and compact nucleic acid into nanoparticles (tens to several hundreds of nm), protect them from degradation and mediate their entry into cells. Cationic lipids and polymers can be used to complex DNA, creating lipoplexes and polyplexes respectively. The use of cationic lipids for gene delivery was first reported by Felgner in 1987 [iii] and lipofection mechanisms are described elsewhere ([iv]).
Synthetic polymers were also extensively studied principally due to their chemical versatility that “easily” allows generating, modifying and synthesizing linear, branched or dendritic polymeric structures with multiple functions[v].
Cationic polymers play a crucial role for the development of gene transfer agents due to their extraordinarily good potential to condense DNA [vi]. As a result, cationic polymers hold great promises for gene delivery and one of the most used polymers for gene delivery was polyethylenimine (PEI)[vii]. Numerous drawbacks of PEI have limited its application and many alternatives (polylysine, polyamidoamine, dendrimer, polyallylamine and methacrylate/methacrylamide polymers) have been synthesized gaining ground on efficacy and reducing toxicity without however reaching all the promises.
One of the main issues remains the activation of innate immune response induced by gene delivery system[viii]; trying too hard to mimic virus physiology can result in reaching the dark side. Gene delivery is sensed as a viral or bacterial attack by the cell that answers by disrupting foreign nucleic acid, inactivating transgene expression or undergoing apoptosis. The overall efficiency is thus lowered.
Up to now, no real breakthrough has emerged in the cationic polymer field.
Helix-IN DNA transfection reagent, our new CHAMP polymer, presents all the characteristics of a classic cationic polymer enhanced with a nucleic acid shielding core to lower cellular stress and combined with a biodegradable property; which lead to an enhanced transfection.
PRINCIPLE
BI-FUNCTIONAL DUAL COPOLYMER STRUCTURE: DNA PACKAGING
The particularity of this novel CHAMP technology comes from the fact that the bi-functional cationic biopolymer is made up of three moieties, each bearing different characteristics and functions.
- The first part in the vicinity of the polymer binds and condenses DNA to an unprecedented level and contributes to cytosol delivery.
- The second component is a pH responsive and cleavable linker that improves cellular delivery by favoring endosomal membrane destabilization.
- The third moiety with a defined and optimized molecular weight serves as a DNA shield and nuclear uptake facilitator.
The molecular weight and length (unique for each type of polymer) of each moiety are important parameters linked to overall transfection efficiency.
Helix-IN, our new bi-functional polymer-based transfection reagent differs from others virus-like vectors: DNA is hidden from the cell until its delivery to the nucleus.
How does it work
1- PROTECTION and SERUM STABILITY
The design of Helix-IN, our new kind of CHAMP polymer allows the (1) positively charged polyplexes to be stable in solution and (2) not to aggregate overtime.
The structure, polyamine composition & grafting density of the CHAMP polymers were finely tuned and optimized to place the polyplexes at the exact interface where solubility is not affected over time. Moreover, hydrophilic groups were ingeniously arranged within the polymer to lower interactions with negatively charged serum proteins (albumin…) for a more efficient gene carrier definition.
Polyplexes remains intact and DNA is protected from degradation…
This positively charged bi-functional polymer presents enhanced DNA-binding properties allowing extent protection of DNA; the positive DNA/polymer charge ratio keeps DNA bound to polymer, playing a key role in protecting nucleic acid from degradation by serum enzyme. We designed this polymer so that no DNA degradation is observed even when incubated in 50% fetal calf serum at 37°C for 24H.
2 - CELLULAR UPTAKE
Cationic complexes bind to cell membrane mainly through electrostatic interactions (figure 1 – 1) and most polyplexes are taken up by the cell through endocytosis pathways (macropinocytosis, phagocytosis, endocytosis). One of the most documented routes of endocytosis is mediated by clathrin (figure 1 – 2). Once endocytosed, complexes are internalized in an early endosome where pH drops from 7.4 (cell surface) to 6.0 (lumen of endosome). The pH will drop to 5 as the endosome progresses to its late phase.
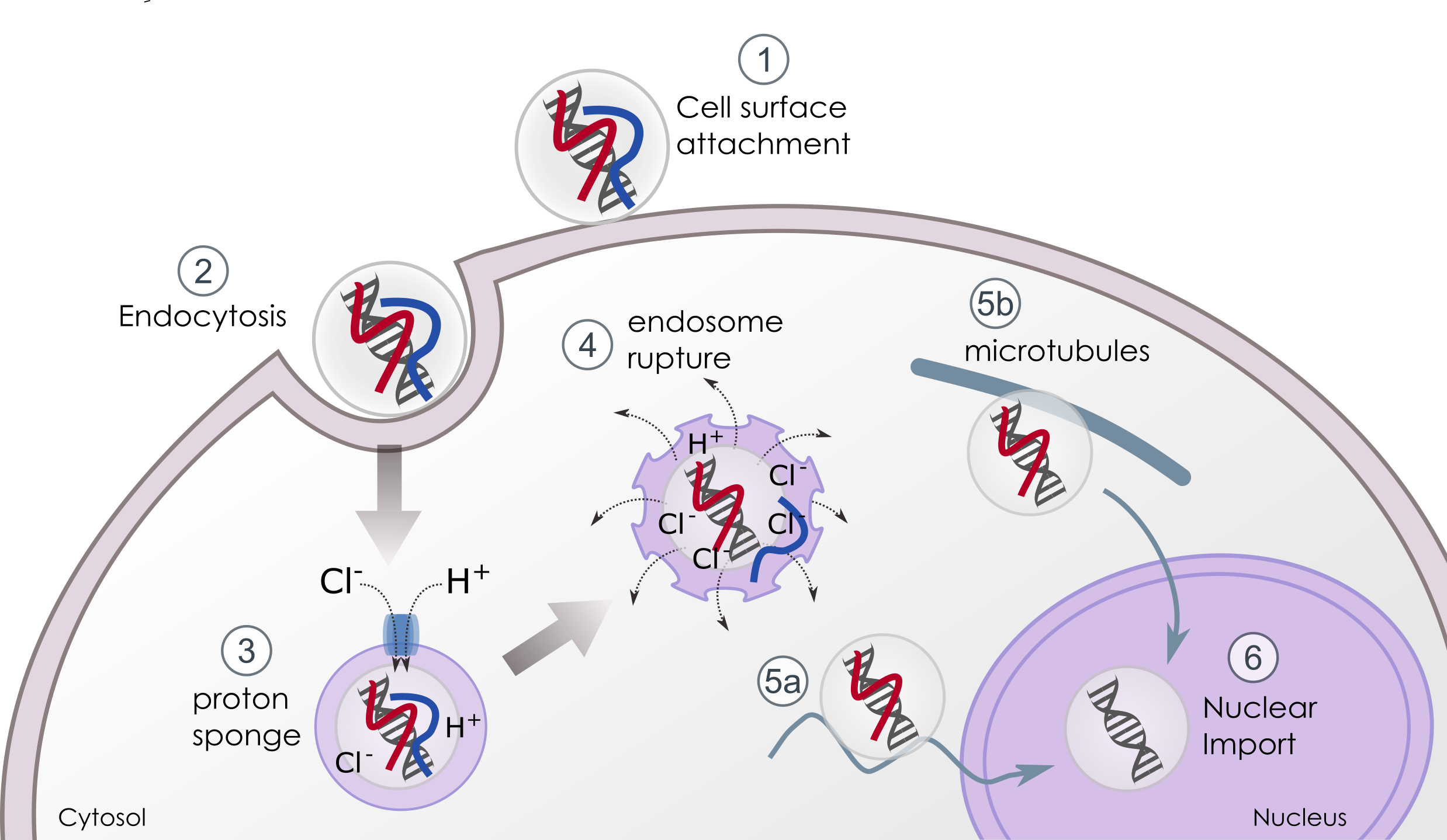
Figure 1. Intracellular trafficking of polyplexes. Excess of polycations at the surface of the polyplex allows attachment to the cell surface (1) and uptake or internalization by the target cell, generally through endocytosis (2). Once into endosome vesicle (3), higher degree of protonation of the proton-sponge polymer causes influx of ions and the pH responsive linker is cleaved, releasing the first polymer unit (blue). The increase of osmotic pressure that leads the vesicle to swell and rupture is favored by the exposition of the hydrophobic domain of the linker (4). The third unit (red) remains bound to the nucleic acid thus lowering the sensing of the DNA by the cell and assisting its nuclear delivery through direct import into the nucleus (5a) or via microtubules trafficking (5b). Once inside the nucleus (6) the DNA is then expressed.
3- ENDOSOMAL ESCAPE & DNA RELEASE
Polyplexes evade endosome and release their cargo into nucleus through the cationic polymer buffering capacities related to the “proton sponge” effectix.
The protonable amines acting as weak base in acidic medium destabilizes pH inside the endosome: once inside the endosomes, specific ATPases generate an influx of protons that are buffered by the polymer (figure 1 – 3). The massive and continue flow of protons is accompanied with passive entry of chloride ions that results in accumulation of water. As a consequence, the vesicles swell until endosomal rupture and their content is delivered into the cytosol (figure 1 – 4).
The first polymeric block plays this role. Moreover the pH responsive and cleavable hydrophobic part adds supplementary features. Indeed, the linker hidden at physiological pH gets exposed at acidic pH. This leads to its cleavage and to the hydrophobic zone exposition which promote endosomal membrane fusion/destabilization.
At this stage, several important pitfalls can impair transfection efficiency:
- The capacity of DNA to escape from endosomes is one of the major limitations of the transfection
- It is generally admitted that once delivered into cytosol, DNA must rapidly be imported in the nucleus to avoid cytosolic degradation
- The presence of cell sensors in endosomes (also on cell surface) that can recognize foreign nucleic acids and induce a protective response inhibiting transfection
Bi-functional co-polymer: inside the cell, the first block that compacts the DNA presents high buffering capacities that favor endosomal swelling, the pH-responsive cleavable linker promotes membrane destabilization and releases of stealth DNA into the cytosol; the third block protect and guide DNA into nucleus.
4- TRANSPORT & NUCLEAR INTERNALISATION
Once released from endosome, polyplexes have to migrate to the nucleus either via microtubules (figure 1 – 5a) or through nuclear import machinery (figure 1 – 5b). In general, large DNA molecules (>3000bp) and polyplexes remain almost immobile as diffusion is size-dependent into the cytoplasm[ii] and numerous cytosolic nucleases degrade nucleic acids. Being still complexed to the third moiety of our bi-functional polymer, the smaller positively charged polyplexes can interact with anionic microtubules or motor proteins, or diffuse in a stealth mode until their nuclear uptake. During all these procedures, the DNA is masked and protected from degradation.
The most evident way of nuclear entry for immortalized cells, is during mitosis where redistribution of cellular material occurs and nuclear membrane is disrupted, however, not all the cells follow a proliferative pattern. Up to now, little is known on nuclear import of polyplex vectors. As soon as DNA has reached the nucleus, it is released from the second part of the polyplexes whom positive charges, molecular mass and grafting design where designed to improve transfection.
WHAT ARE THE APPLICATIONS?
Helix-IN™ DNA Transfection Reagent
The principal use is DNA transfection for in vitro and in vivo applications.
The CHAMP technology increases transfection: more DNA enters the cells and DNA is addressed to the nucleus in a stealth mode without alerting and stressing the cells…This reagent is ideal for immortalized cell lines preferentially adherent such as HEK-293, NIH-3T3, CHO, COS, HeLa, MCF7, MEF, RPE, C2C12….
It is perfect for co-transfection of multiple DNA.
In vivo, DNA is condensed and protected into small polyplexes that limit immune responses and are able to navigate through circulatory system until they delivery.
WHAT IS THE PROTOCOL?
The protocol is simple: transfection reagent is directly mix with DNA using ratios 1:1 to 3:1 (1µL per µg DNA to 3µL per µg DNA) depending on the cell type. After 30 min of incubation time, polyplexes and boost are added onto cells.
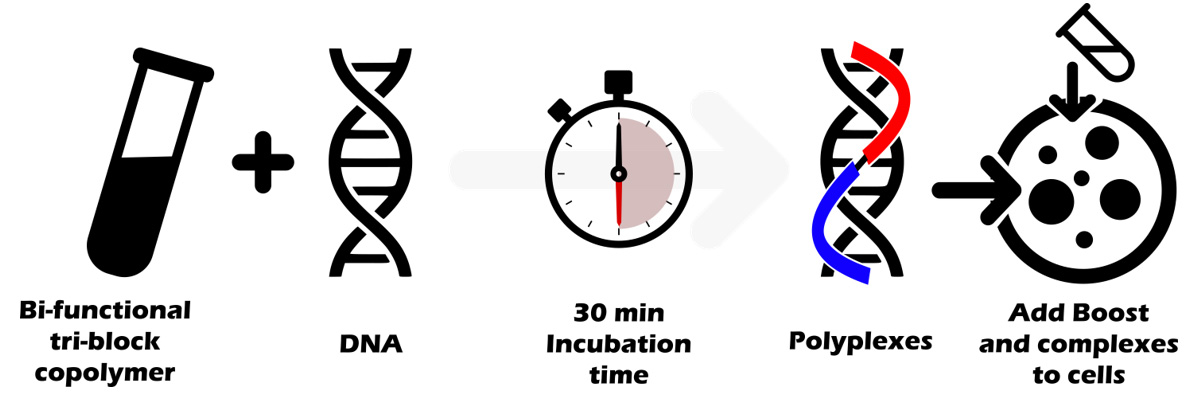 This 30 min incubation time is the cornerstone of the protocol allowing a full compaction and protection of DNA.
This 30 min incubation time is the cornerstone of the protocol allowing a full compaction and protection of DNA.
During the nanoparticles/DNA complexes self-assembly, it is critical to wait at least 30 minutes to enable the co-polymers and DNA to form stable supramolecular nanoparticles. Due to the multipart nature of the copolymer, the time for forming and stabilizing the complexes is slightly longer than with “simple” polymers where complexes formation occurs more rapidly (10-20 min).
WHAT ARE THE MAIN DIFFERENCES BETWEEN LIPOFECTION AND POLYFECTION?
Lipofection and polyfection (respectively lipid-based and polymer-based transfection) are two methods of transfection using synthetic vectors to deliver nucleic acids into cells. Even if the finalities of the two techniques are the same, some differences still exist orienting the nucleic acid delivery applications to one or the other (refer to table below).
[i] Slivac, I. et al (2016). Non-viral nucleic acid delivery methods. Exp. Op. Biol Ther. Vol 17, 2017 - Issue 1
[ii] Xiang, Y. et al (2017). Recent development of synthetic nonviral systems for sustained gene delivery. Drug Discov Today. 22(9):1318-1335.
[iii] Felgner, PL. et al (1987). Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 84(21):7413-7.
[iv] Cullis, PR. et al (2017). Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol Ther. 5;25(7):1467-1475.
[v] Prabu, SL. et al (2017). Biopolymer in Gene Delivery. Intech book Chapter 7. DOI: 10.5772/65694
[vi] Ruponen, M. et al (1999). Interactions of polymeric and liposomal gene delivery systems with extracellular glycosaminoglycans: Physicochemical and transfection studies. Biochimica et Biophysica Acta: Biomembranes, vol. 1415, no. 2, pp. 331–341.
[vii] Boussif, O. et al. (1995). A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7297-301.
[viii] Sakurai, H. et al. (2005). Innate immune response induced by gene delivery vectors. Int J Pharm. 354(1-2):9-15.
[ix] Kim, TI. Et al. (2011). Bioreducible polymers with cell penetrating and endosome buffering functionality for gene delivery systems. J Control Release. 152(1):110-9.
[x] Luby-Phelps K (1987). Hindered diffusion of inert tracer particles in the cytoplasm of mouse 3T3 cells. Proc Natl Acad Sci U S A. 84(14):4910-3.