BLOG > Publications & Citations > A multichaperone condensate enhances protein folding in the endoplasmic reticulum
Authors: Leder, A., Mas, G., Szentgyörgyi, V. et al.
Source: Nat Cell Biol (2025).
We are thrilled to share insights from a genuine study entitled "A multichaperone condensate enhances protein folding in the endoplasmic reticulum" published in Nature Cell Biology by Anna Leder et al.
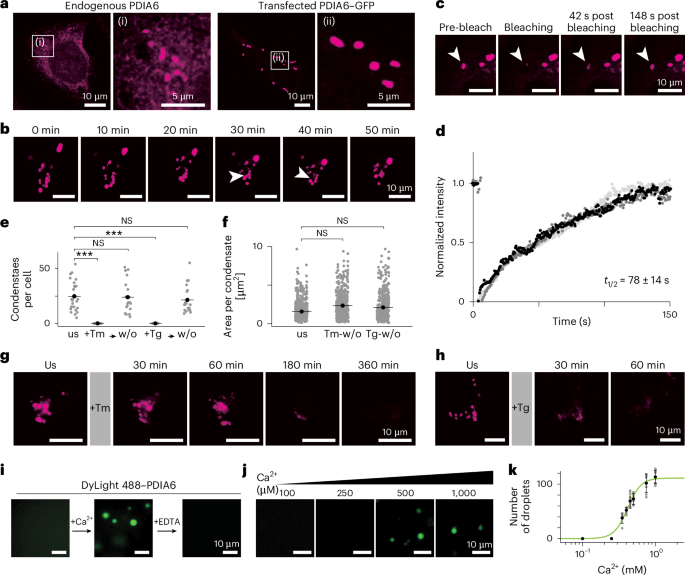
They discovered that the ER protein PDIA6 forms dynamic, calcium-dependent multichaperone condensates, acting as "folding factories" that actively enhance protein folding and prevent misfolding. This crucial self-organized structure significantly impacts protein processing (e.g., proinsulin) and maintains cell viability, revealing a new layer of organization in ER proteostasis.
Congratulations to all the authors on this excellent article!
Our Helix-IN transfection reagent was used to facilitate transient cell transfections in various cell lines (HeLa, HEK293A, U2OS, INS-1) and to generate the stable Halo-tagged PDIA6 HeLa cell line, as well as for CRISPR-Cas9-mediated gene knockout attempts, which further confirmed PDIA6's essential role.
Read the article Check Helix-IN