BLOG > Publications & Citations > Anti-SARS-CoV-2 serology and neutralizing antibodies against Omicron variants
Authors: Dépéry, Léa et al.
Source: Microbiology Spectrum 13:e01568-24
We are thrilled to share insights from a recent study titled "Anti-SARS-CoV-2 serology based on ancestral RBD antigens does not correlate with the presence of neutralizing antibodies against Omicron variants" published in Microbiology Spectrum by Dépéry, Léa et al.
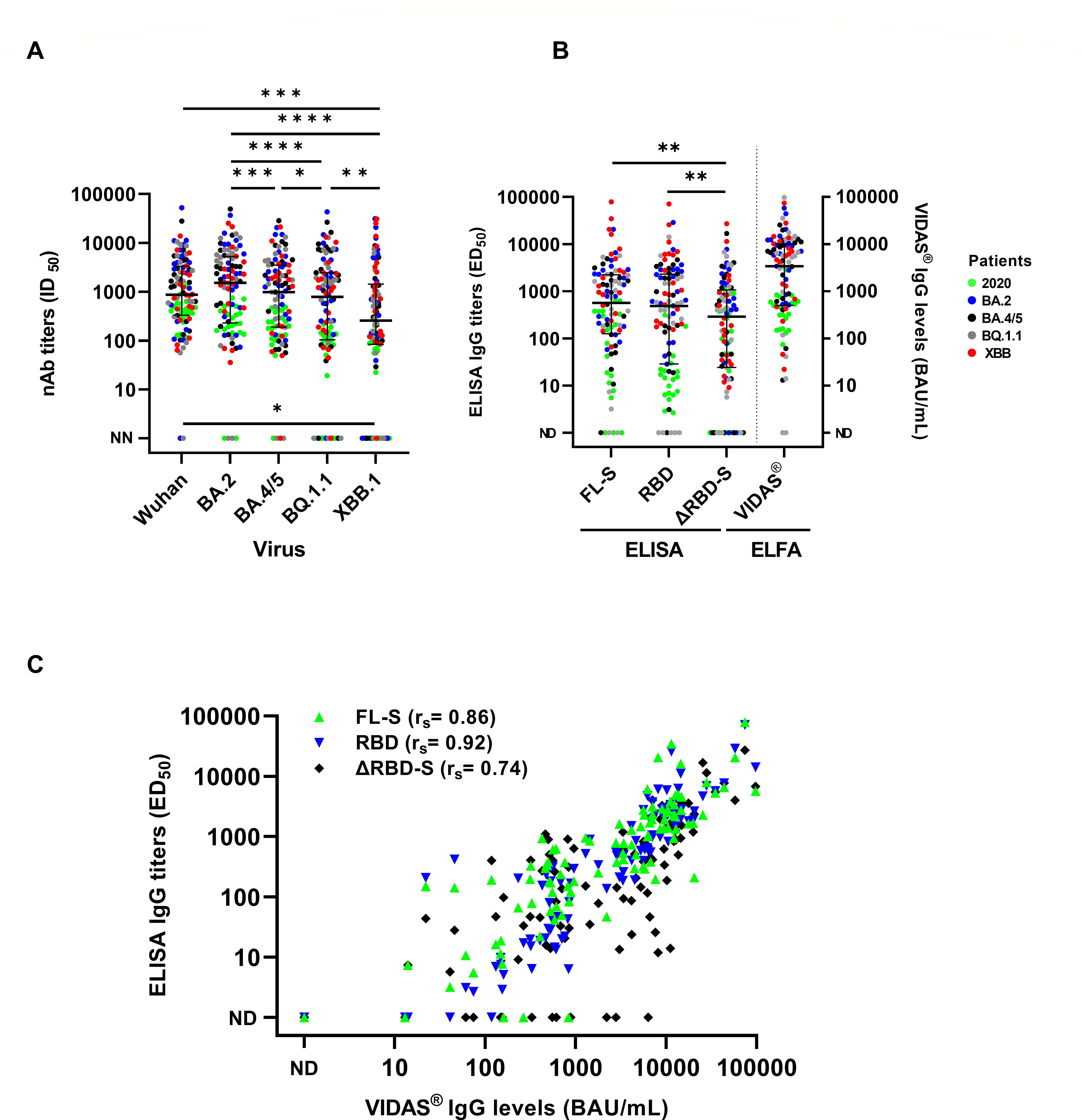
"Neutralizing antibody titers and binding antibody levels are considered correlates of protection against severe SARS-CoV-2 infection. The clinical utility of serology should be reevaluated in light of the emergence of escape variants, as commercial antibody-binding assays have not been adapted to the virus’ antigenic evolution. We compared anti-SARS-CoV-2 antibody titers in four quantitative serological tests based on variable ancestral spike antigens (three in-house ELISAs and the prototype VIDAS SARS-CoV-2 IgG QUANT assay) and neutralization assays against the pseudotyped Wuhan, BA.2, BA.4/5, BQ.1.1, and XBB.1.1 viruses in a cohort of 100 patients infected in 2020 or during the Omicron waves. Binding antibody levels correlated well with neutralizing antibody titers for Wuhan, BA.2, and BA.4/5, but the association decreased for BQ.1.1 and XBB.1 (for the VIDAS assay, Spearman’s correlation was 0.82 [95% CI 0.74–0.88] and 0.61 [0.46–0.72] for BA.2 and XBB.1, respectively). In 15% of patients with no neutralizing antibodies against XBB.1, the VIDAS assay still yielded binding antibody levels ranging from 74 to 7,652 binding antibody units/mL. Using an adjusted threshold based on receiver operating characteristic (ROC) curve analysis, the specificity of neutralizing antibody detection increased from 0.15 (95% CI 0.02–0.45) and 0.17 (0.04–0.41) to 0.92 (0.64–1.00) and 0.83 (0.59–0.96) against BQ.1.1 and XBB.1, respectively. Serological tests based on receptor-binding domain antigens from the ancestral virus fail to predict neutralizing activity against the latest circulating Omicron variants. Adapting serological tests may improve their clinical utility in immunocompromised patients."
Congratulations to all authors for this great article.
Our Helix-IN transfection reagent was used to produce Pseudo-typed SARS-CoV-2 virion in HEK293T cells. And our Luciferase Assay Kit was used to measure Luciferase activiry in HeLa cells.
Read the article See our Helix-IN See our Luciferase Kit