BLOG > Publications & Citations > Impact of pre-existing anti-polyethylene glycol (PEG) IgM on biodistribution
Authors: Shunji Abe et al.
Source: Journal of Controlled Release, Volume 383, 2025, 113821.
We are thrilled to share insights from a recent study entitled "Impact of pre-existing anti-polyethylene glycol (PEG) IgM on biodistribution and humoral response of intramuscularly administered PEGylated mRNA loaded lipid nanoparticle" published in Journal of Controlled Release by Shunji Abe et al:
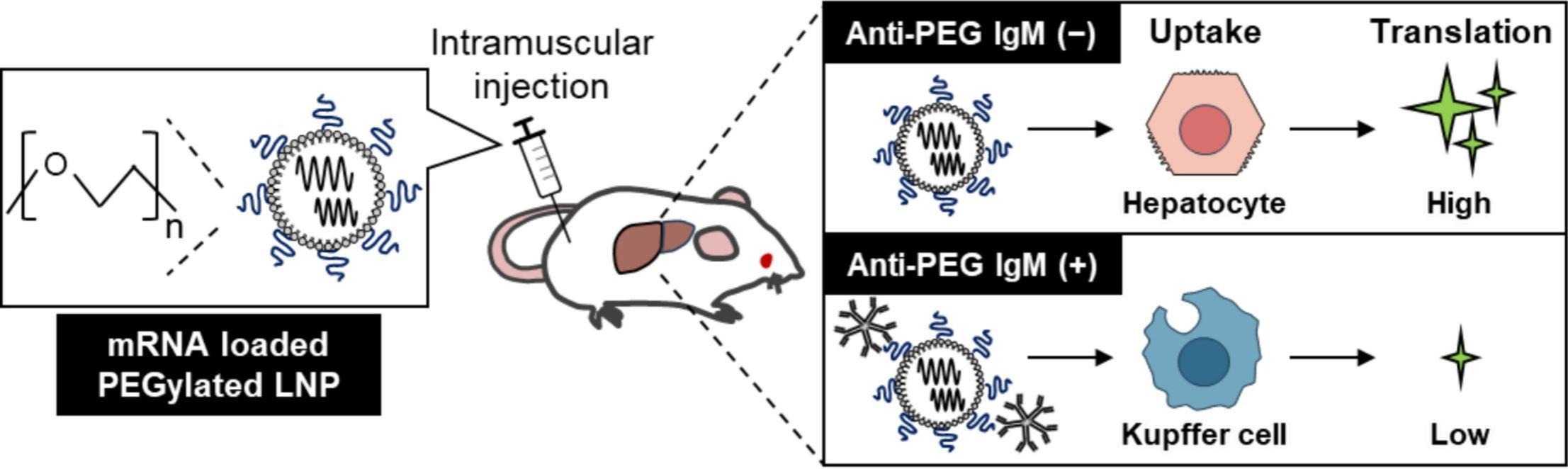
"With the approval of mRNA vaccines for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), lipid nanoparticles (LNP) have emerged as a powerful tool for nucleic acid delivery. Modification of LNP with polyethylene glycol (PEG)-lipids contributes to their in vitro and in vivo stability by reducing aggregation of the particles. Despite the general acknowledgement of the low immunogenicity of PEG, there are numerous reports of the occurrence of anti-PEG antibodies in the blood of healthy individuals. Furthermore, there are reports that pre-existing anti-PEG IgM antibodies attenuated the efficacy of PEG-modified drugs administered systemically. Skeletal muscles, the administration site for mRNA vaccines, are highly vascularized by a network of blood vessels to provide them with nutrients and oxygen. Since skeletal muscles can contain circulating anti-PEG antibodies or the administered mRNA-LNP extravasate into bloodstream, the purpose of this study was to evaluate the effects of pre-existing anti-PEG IgM on the protein translation, biodistribution, and humoral responses to mRNA-loaded LNP (mRNA-LNP) administered intramuscularly (i.m.) in mice. We found that the presence of anti-PEG IgM in blood has only a minor effect on the translation and distribution of mRNA-LNP in the localized muscle area where the mRNA-LNP were administered. Circulating anti-PEG IgM did not increase the total accumulation of mRNA-LNP in the liver, but did markedly diminish its protein translation because the mRNA-LNP were delivered primarily to Kupffer cells rather than to hepatocytes. Binding of anti-PEG IgM to mRNA-LNP, and subsequent complement activation, suppressed mRNA translation loaded in LNP in the liver. Repeated intramuscular injections of SARS-CoV-2 Spike protein mRNA-LNP elicited a robust immune response. Our results indicate that the presence of circulating anti-PEG IgM does not interfere with the efficiency of mRNA vaccines administered i.m. in mice. We can speculate that non-specific translation of mRNA vaccines in somatic cells may be inhibited in vivo by circulating anti-PEG IgM, reducing adverse effects such as hepatitis and myocarditis that are caused by immune responses against translated antigen proteins."
Congratulations to all authors for this great article.
Our Spike SARS CoV-2 mRNA was encapsulated in LNP injected in mice.
Read the article See our Spike SARS CoV-2 mRNA