BLOG > Technologies & Applications > Magnetofection-Mediated Gene Delivery in Mature Primary Neurons: A Comprehensive Analysis of NeuroMag Performance in Long-Term Cultures
Abstract
Efficient gene transfer into post-mitotic primary neurons remains a significant technical challenge in neuroscience research. We present a comprehensive analysis of Magnetofection using NeuroMag reagent based on hundreds of publications spanning from 2007 to 2026. Our analysis focuses on transfection efficiency in mature neuronal cultures (≥10 days in vitro, DIV), where conventional methods typically fail. NeuroMag demonstrated efficient and consistent transfection rates in mixed and pure neuronal populations with successful genetic modifications of cultures up to 22 DIV. Importantly, Magnetofection preserved neuronal viability, morphology and functional properties enabling long-term expression studies (5-10+ days post-transfection). These findings establish magnetofection with NeuroMag as a reliable method for transfection in primary differentiated neurons, particularly for synaptic and electrophysiological studies requiring mature neural networks.
Keywords: magnetofection, primary neurons, long-term culture, neuronal transfection, gene delivery, neuroscience, NeuroMag
Introduction
Primary neurons represent one of the most recalcitrant cell type for exogenous gene delivery (Washbourne and McAllister, 2002). The difficulty becomes particularly challenging for mature neuronal cultures (≥10 DIV) when synaptic networks have formed, and neuronal function is most physiologically relevant. As terminally differentiated, post-mitotic cells, neurons lack the mitotic machinery that facilitates DNA uptake in dividing cells. Not only, but also their complex morphology with extensive dendritic and axonal arbors further complicates transfection. Moreover, primary neurons are exceptionally sensitive to any perturbations in their environment, making them vulnerable to the chemical and physical stress inherent to most transfection protocols.
Several non-viral transfection methods have been applied to neurons with limited success, and they all show marked declines in their relative efficiency with increasing culture age. Calcium phosphate was among the earliest method used for neuronal transfection. This technique forms calcium phosphate-DNA precipitates that are taken up by cells via endocytosis. These precipitates are large and heterogeneous causing physical stress, interfere with neuronal health and consequently result in low efficiency and poor reproducibility¹. Lipid-based transfection or Lipofection uses cationic lipids to form liposomes that encapsulate DNA and fuse with cellular membranes. Even if improvement were achieved compared to Calcium-Phosphate, cationic lipids are inherently cytotoxic to neurons causing membrane disruption and oxidative stress; efficiency dramatically declines with culture age after 7 DIV². Electroporation or Nucleoporation that delivers DNA by creating transient pores in cell membrane using electric pulses requires cell dissociation thus limiting its use to freshly prepared culture³ – neurons dissociation leads to destroying established synaptic networks and can only be performed on freshly isolated cells (day 0) making it impossible to transfect mature neurons. Moreover, membrane damage results in considerable cell death (~60%).
Magnetofection: driving neuron transfection with magnetic force
Magnetofection was developed to overcome the key limitations of conventional transfection methods: the slow sedimentation of DNA cargos onto cells by gravity alone and the high toxicity. When the cells are placed onto a magnetic plate, the specific magnetic field concentrates the complexes onto their surface within minutes, achieving complete sedimentation in 20 min.
The action of the magnetic force clusters the complexes and favors the cellular entry into endosomes through classic endocytosis pathways such as clathrin or caveolin. At this point, no hole is created leaving the cells membrane intact as opposed to other physical methods of transfection such as electroporation. Once inside the cells, the complexes are then located into endosomes from where they evade due to the action of proton sponge effect. Magnetic beads will remain sequestred into vacuoles, excreted out or be degraded by the cell’s iron metabolism. Depending on its nature, nucleic acid will then be directed to nucleuc through cytoplasmic trafficking (DNA) or remain in the cytoplams (RNAs), (Figure 1).
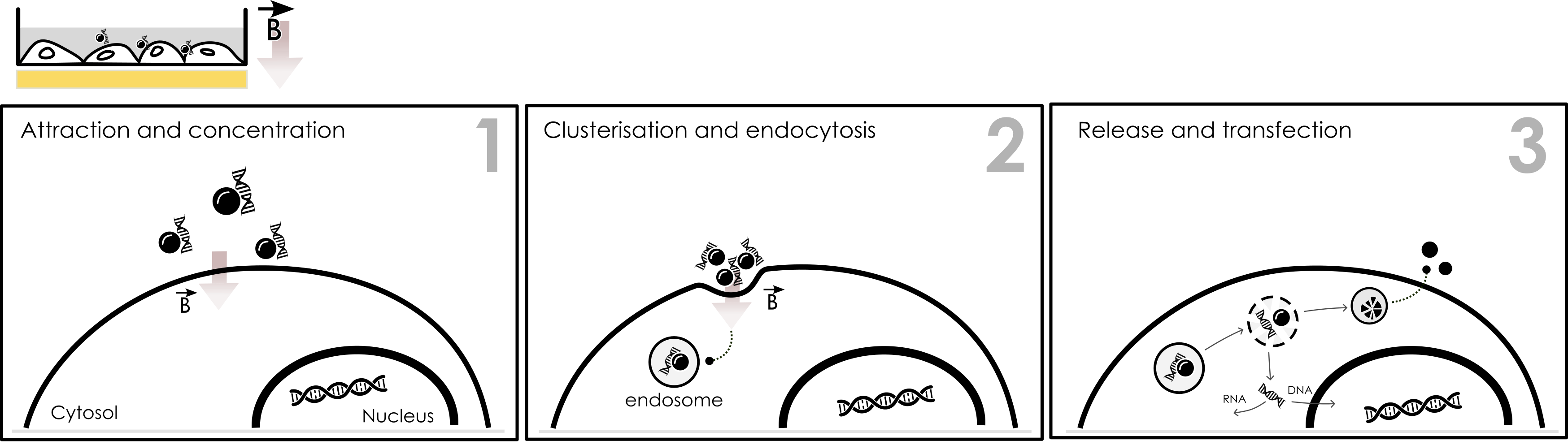
Figure 1: Intracellular mechanism of Magnetofection. Under the influence of a specific magnetic field (B vector) developed by our magnetic plate, the magnetic complexes are attracted and concentrated onto the cell surface (1). The magnetic nanoparticles are clustered and enter the cell through classic endocytosis pathways (2). After evading endosome, nucleic acid is directed to its desired location while magnetic nanoparticles are excreted or remain in vacuole to be degraded.
This method of transfection provides several advantages regarding transfection of sensitive neurons:
(1) Rapid concentration reduces total exposure time of toxic transfection reagents from hours to minutes, thus improving the viability.
(2) The magnetic force covering the entire surface ensures an even and adequate local concentration of DNA complexes at each cell’s membrane.
(3) The physical mechanism of transfection is gentle compared to electroporation involving no membrane disruption.
NeuroMag: Optimized Magnetofection for neuron transfection from 0 DIV to 22 DIV.
NeuroMag is a magnetofection reagent exclusively formulated for primary neurons and neuronal cell lines. This reagent consists of proprietary magnetic nanoparticles with specific patented coating optimized to minimize toxicity in post-mitotic cells while maintaining efficient nucleic acid binding and cellular uptake. NeuroMag enables reproducible transfection of neurons across a wide range of neuronal subtypes and culture ages (figure 2).
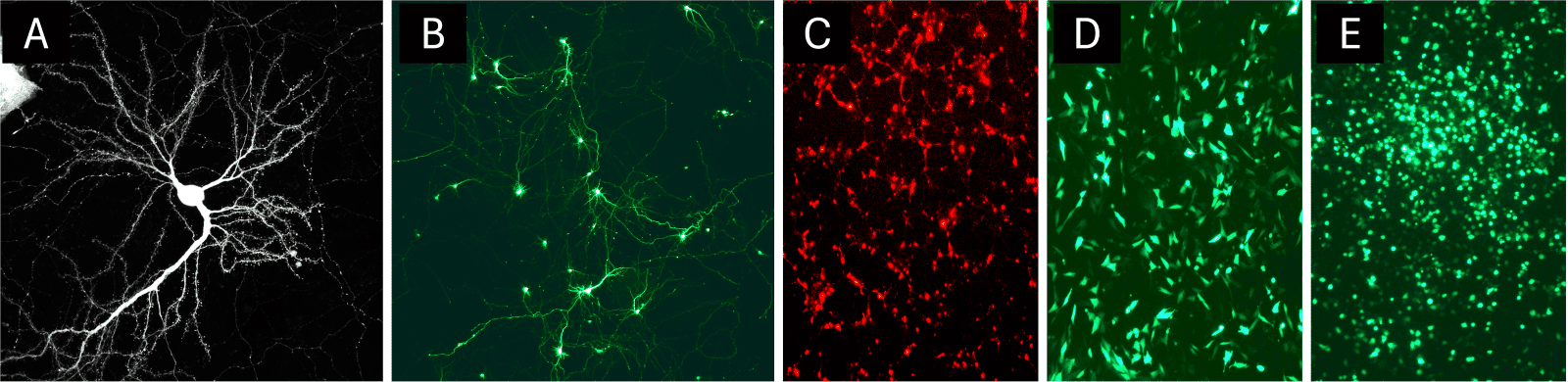
Figure 2: Example of neural cell types transfected with NeuroMag. NeuroMag can adress various neuronal cell types such as primary mouse neurons at DIV 20 (a, image kindly provided by Cécile Charrier⁴), primary rat hippocampal neurons (b), neural stem cells (c), embryonic mouse hypothalamus cell line (d) or microglia cells (e).
When considering long-term cultures from 10-22 days in vitro, the time window where synaptic networks are functionally mature, one of the key parameters for an efficient transfection is the conditions under which neurons are cultured. A rapid protocol can be found at Protocol.io.
a. Set-up the perfect conditions for an efficient magnetofection-based transfection in long-term cultured neurons.
Primary neuron culture is a three-part system consisting of a support (glass coverslip), cells and culture medium and each element has a great role to play for an efficient transfection of long-term neurons and must be dealt with great precaution.

Glass coverslips are essential for neuronal adherence; their surface is coated with poly-L-lysine. The initial steps to prepare the glass require nitric acid incubation (1% vol/vol) overnight following by sonication and sequential ethanol baths. The coverslips are then incubated overnight with poly-L-lysine. Rigorous procedures of coating and washing are crucial for neuron culturing since residual poly-L-lysine is toxic for the neurons.
Culturing cell type of interest a.k.a. primary neurons is without any doubt the most sensitive part of the experience. Dissection and collection of the tissue of interest must be done in accordance with institutional and national guidelines for animal experimentation and numerous critical steps can be seen as various pitfall for cell survival. Precise and correct dissection, dissection buffer temperature (2-3°C), Nu-serum composition, and incubation time with the enzyme to dissociate neurons are all sensitive factors that require special care. For late transfection, a higher cell density is necessary (800.000 cells per 35 mm dish).
It is recommended to change 50% of the medium with feeding medium…
Finally, managing the culture medium throughout the cultivation of neurons up to 22 DIVs is one of the most important factors in ensuring the well-being of cells, thereby favoring magnetofection. The culture medium should be replaced gradually with a feeding medium containing B27. At 5 DIV, 50% of the medium should be changed and so on, every 3 days. The composition may vary but the following feeding medium can be used: MEM supplemented with 15 mM, HEPES, 0.45% (wt/vol) D-glucose, 1 mM sodium pyruvate, 2 mM L-glutamine, and 2% (vol/vol) B27. Finally, 24 hours before Magnetofection half of the culture medium should be replaced with fresh medium.
These favorable culture conditions were first systematically characterized by Buerli et al. in Nature Protocols, demonstrating successful transfection of rat hippocampal neurons from 0 to 21 DIV using Magnetofection⁵. This landmark publication established magnetofection as a viable alternative to viral transduction for studies requiring transient expression in mature neurons.
b. Magnetofection protocol for primary neurons.
In the following, a brief summary of NeuroMag protocol that can be followed to transfect neurons in 24-well plate (figure 3):
- Prepare a DNA solution by diluting 1 µg plasmid into 100 µL OptiMEM – medium should not contain any supplement (serum, antibiotics, growth factors…).
- Prepare a tube containing 3.5 µL NeuroMag.
- Add DNA solution to NeuroMag magnetic nanoparticles.
- Incubate 20 min at room temperature.
- Add complexes to the cells in a drop-wise manner.
- Place the cells onto magnetic plate.
- Incubate the cells 20 min under standard culture conditions.
- Remove the magnetic plate.
- Cultivate the cells under standard culture conditions until evaluation of the transgene expression (24 H to 7 days).
Complete information, original protocol as well as optimization procedures can be found on our website at: NeuroMag transfection reagent.
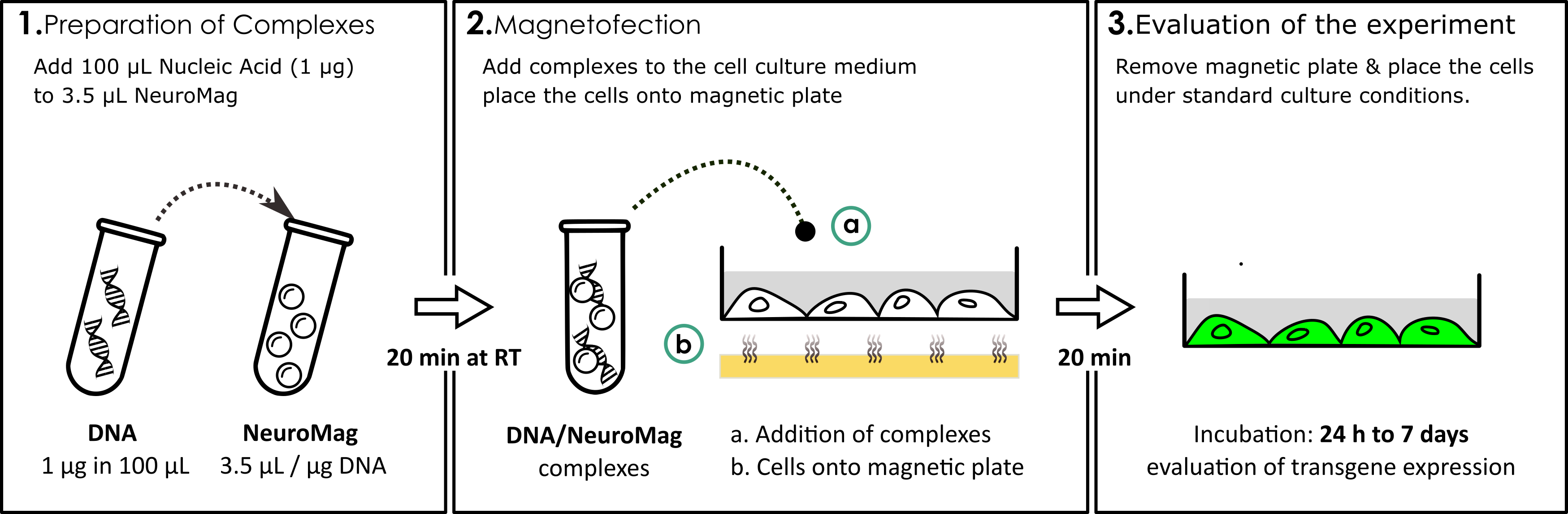
Figure 3: Example of NeuroMag protocol for a 24-well plate. DNA solution is added to NeuroMag transfection reagent at a 3.5:1 ratio. After 20 min incubation time at RT, the complexes are added into the cell supernatant (a) and the cell culture plate is placed onto magnetic plate (b). After 20 min incubation (Magnetofection), the cells are removed from the magnetic plate and incubated under standard culture conditions until evaluation of the experiment (24 H to 7 days).
Review of the literature on neuronal transfection
This article presents a systematic analysis of NeuroMag performance based on about a hundred of peer-reveiwed publications from 2008 to 2026 that utilized this reagent for neuronal transfection. We focus specifically on applications in cultures ≥ 10 DIV, analysing transfection efficiency across neuronal subtypes and culture ages, cell viability and morphological preservation, duration and stability of transgene expression. Following compatible applications are also studied such as imaging, electrophysiology and biochemistry. When available, we extracted cellular parameters including species (rat, mouse…), brain region and culture age at transfection (DIV) and technical parameters such as DNA amount, incubation time and magnetofection experimental procedures.
a. Repartition.
Regarding species distribution, the predominance of rat neurons reflects their traditional use in neurobiological research, though mouse neurons have gained increasing prominence in recent years due to the availability of genetic models (figure 4a).
The distribution of studies by culture age at transfection revealed two main age categories: 10-14 DIV and 15-20 DIV that reflects experimental paradigm in neuroscience (figure 4b). The 10-14 DIV window represents early mature cultures with developing synaptic networks ideal for studying synaptogenesis and early network formation. The 15-20 DIV window captures fully mature cultures with established synaptic connections, appropriate for studies of synaptic plasticity and network function. Notably a non-negligeable proportion of studies successfully transfected neurons at high DIV approaching the upper limit of typical in vitro culture longevity and demonstrating the robustness of magnetofection even in the oldest sustainable culture.
The analysis revealed successful transfection of diverse neuronal subtypes. Hippocampal neurons were used in the majority of studies making them the most commonly employed cell types with both CA1/CA3 pyramidal cells and dentate granule cells represented. Cortical neurons are the other main category with predominantly layer 2/3 and layer 5 pyramidal cells. Cerebellar neurons, primarily Purkinje cells and granule cells and spinal neurons including motor neurons and dorsal horn neurons count for the two last most cited categories (figure 4c).
Specific neuronal subpopulations that are also represented in figure 4c were also successfully targeted such as GABAergic interneurons or pyramidal neurons.
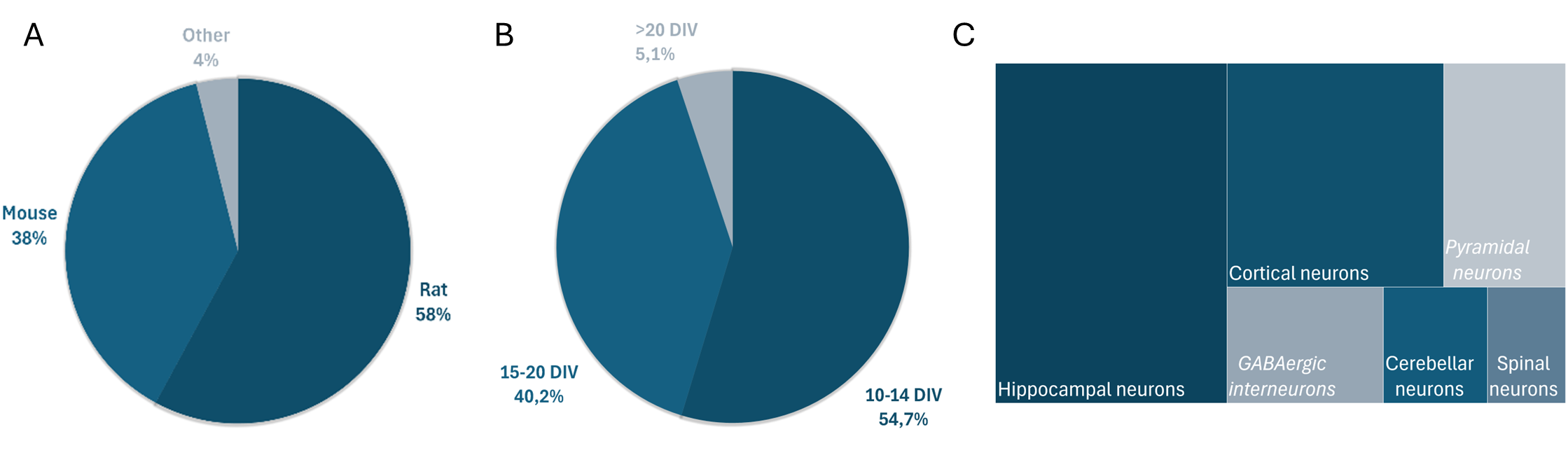
Figure 4: Repartition of literature. Review of literature allows to draw the distribution of species (a), age of cultures (b) and territories (c). In (c), italic names represent neuronal subpopulations described in the publications.
b. Protocol parameters.
Analysis of methodological details across publications revealed strong consensus on optimal parameters (figure 5). For DNA quantity per well or dish, 12-well plates containing 18mm coverslips typically used 0.5-1µg with a modal value of 1.0µg; 35-mm dishes or 6-well plate used 1-3.0 µg with a modal value of 1.5 µg.
Magnetic field exposure duration ranged from 15-30 minutes with a modal value of 25 minutes. Notably, longer exposure exceeding 30 minutes did not improve efficiency while shorter exposure of less than 15 minutes reduced efficiency. This confirm an optimal window where sufficient time is provided for complex-cell interaction without prolonged exposure to potentially toxic components.
The ratio of transfection reagent to DNA showed also consistency across studies. If early culture neurons are more prone to transfection using standard ratios of NeuroMag as described in the protocol (3 µL per µg or 3.5 µL per µg), as DIV increase it appears that lower ratios of NeuroMag induce better efficiency: ratios of 1-2 µL per µg DNA. These ratios appear to balance the need of adequate complex formation with the desire to minimize exposure to transfection reagent; and that's where Magnetofection comes into its own.
Post-transfection, medium handling was also standardized. The optimal approach involved 50-80% replacement of culture medium. Importantly, complete culture medium removal increased toxicity presumably by completely removing protective factors and exposing neurons to abrupt environmental changes. On the other hand, efficiency was reduced when the medium was not changed, likely because of residual transfection complexes in the supernatant could continue to interact with cells in uncontrolled ways.
Cell density at initial plating correlated with the intended transfection timepoint. For transfection at 10-14 DIV, optimal initial density was 50.000-200.000 cells per cm², for latter transfection (>15 DIV), higher initial density of 100.000-400.000 cells per cm² was preferred. This accounts for natural cell loss during maturation, ensuring adequate density remains at the time of transfection, confirming the initial protocol by Buerli et al.
Finally, the most adequate composition for the culture medium is MEM medium supplemented with Nu-serum (10-15%) and 2% B27. This would lead to optimal neuron culture and allow detection as soon as 16 to 24 hours.

Figure 5: Protocol parameters. Summary of the conditions favorable to magnetofection in long term neuron cultures.
c. Cell viability and morphology.
Among the studies reporting quantitative viability data, immediate post-transfection viability (0-24 hours) averaged 92 ± 4% compared to 95 ± 3% in untransfected controls indicating minimal toxicity. At 48 hours post-transfection, viability was 88% ± 5% and was still elevated after 5 days (78% ± 8%) demonstrating sustained health in the surviving population (figure 6).
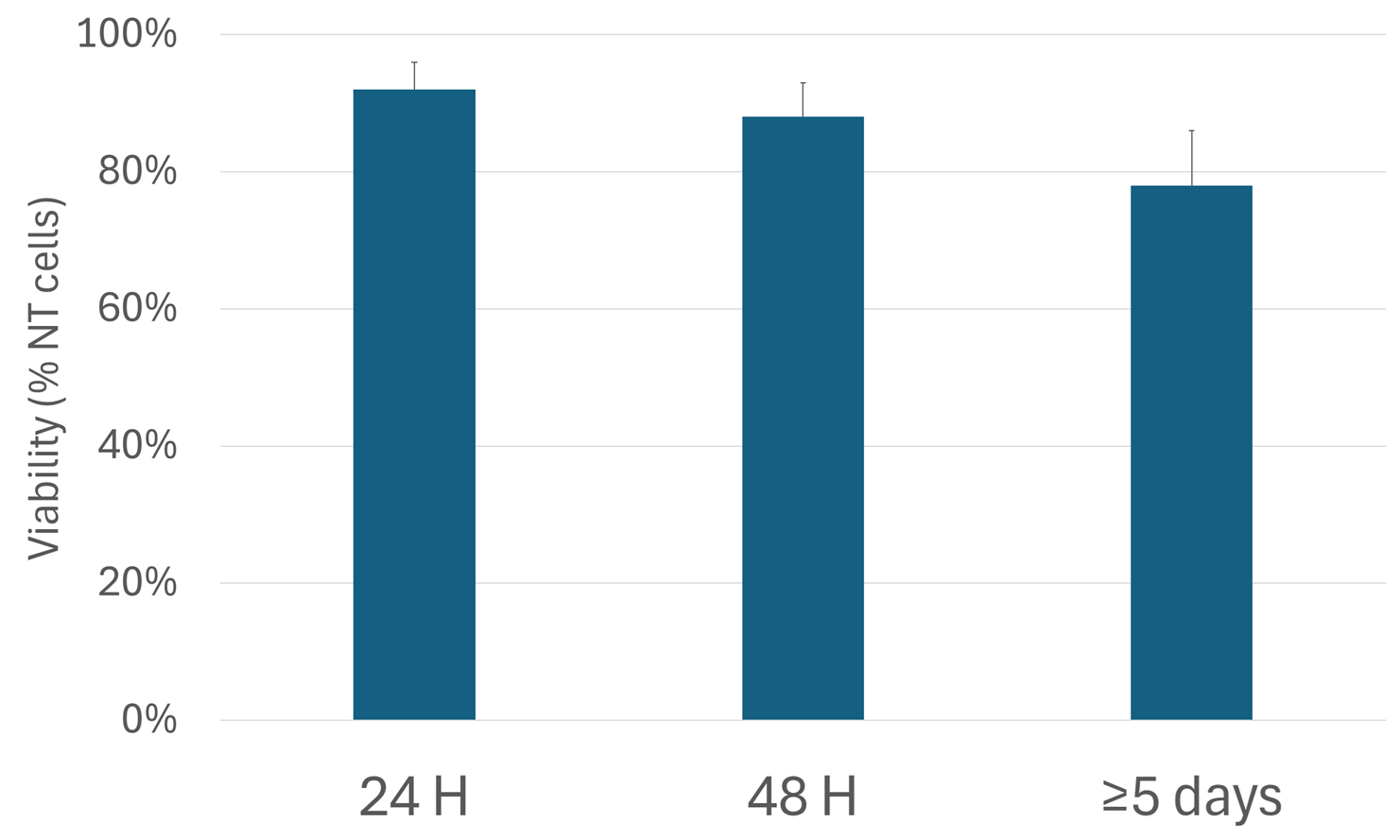
Figure 6: Neuronal survival after transfection. Reporting of viability index of neurons transfected with NeuroMag.
All studies examining neuronal morphology reported preservation of key structural features. Dendritic arbor complexity as assessed by Sholl analysis, remained unchanged compared to untransfected controls. Spine density on transfected dendrites was maintained at normal levels. Axonal projection, patterns developed normally and synaptic puncta density, measured using pre- and postsynaptic markers, showed no significant differences.
A critical observation from Buerli et al. and subsequent studies demonstrated that GABAergic synapse formation proceeded normally in neurons transfected at 6-10 DIV and examined at 12 DIV. This indicates that Magnetofection does not disrupt the developmental program, allowing natural maturation process to continue despite the introduction of exogenous DNA.
d. Applications.
The description of experimental techniques applied to magnetofected neurons reveals the versatility of the method. Fluorescence imaging was the most common application, used in most works for both live-cell imaging and immunofluorescence. Protein overexpression studies including receptors, ion channels and scaffolding proteins appear in large part of publications. Electrophysiology using patch-clamp for whole-cell recording is also described in the literature. Other techniques are also described following Magnetofection such as gene knockdown using siRNA or shRNA for loss-of-function studies as well as Western blot or other biochemical approaches for protein expression analysis, calcium imaging or PCR and gene expression analysis (Table 1).
| Application | Description |
| Cellular imaging | Expression of fluorescent proteins for visualization of morphology, vesicular trafficking, and synaptic dynamics |
| Electrophysiology | Patch-clamp on transfected neurons for study of electrical properties and synaptic transmission |
| Overexpression | Expression of proteins of interest (ion channels, receptors, scaffolding proteins) |
| Knockdown (siRNA/shRNA) | Inhibition of gene expression for functional studies |
| Immunofluorescence | Labeling and localization of endogenous and exogenous proteins |
| Western blot and PCR | Biochemical and molecular analysis of transfected neurons |
Table 1: Common applications following NeuroMag transfection.
Among the publications it appears that numerous studies performed double-transfection with two separate plasmids ; maximum total DNA used successfully was 2µg. Applications of double-transfection included two-color imaging for visualizing different cellular compartments or proteins and protein-protein interaction studies.
Conclusion
The consistent performance of NeuroMag across about a hundred publications established Magnetofection as a reliable method for genetic manipulation in mature primary neurons in long-term cultures. This addresses a long-standing methodological limitation in neurosciences: the inability to efficiently transfect neurons after they have formed functional synaptic networks.
The 10-22 DIV window is particularly important because synaptic maturation occurs during this period and many neurodegenerative processes manifest specifically in mature neurons making this age range critical for disease modeling. Conventional transfection methods largely fail in this time window, forcing researchers to either transfect earlier, use virus vectors, or perform experiments in immature cultures with reduced physiological relevance. Magnetofection thus provides direct, efficient, non-viral gene delivery into mature neurons.
The superior performance of Magnetofection in neurons as well as mature neurons comes from several factors:
- The kinetic enhancement provided by the magnetic field concentrates transfection complexes at the cell surface is faster than with diffusion alone. This dramatically reduces the total time of exposure to potentially toxic cationic lipids ; time that can be shortened also by performing a medium change right after the magnetofection procedure.
- The magnetic field ensures that every cell receives adequate complex concentration at each cell’s membrane.
- The magnetic gradient may minimize complex aggregation compared to prolonged diffusion maintaining particle size in the optimal size range for endocytosis, typically 100-200nm.
- Finally, magnetic field forces the magnetic nanoparticles to cluster at the cell surface inducing membrane deformation that favors endocytosis through classic endocytosis pathway. Opposed to electroporation that disrupts membrane, with Magnetofection, no hole is created leaving cell membrane intact favoring viability.
Our comprehensive analysis of about a hundred peer-reviewed publications demonstrate NeuroMag as a robust reproducible method for transfecting mature primary neurons cultured for 10-22 days in vitro. The data revealed consistent transfection efficiency maintained across culture ages, neuronal subtypes and as many different culture conditions as there are publications. Following transfection, neurons maintain viability, morphology and functional properties being compatible with any biological application. After its introduction by Buerli and colleagues, magnetofection with NeuroMag has become nowadays a standard tool in cellular neuroscience enabling experiments that were previously impractical or impossible.
References
¹ Jiang M, Chen G. High Ca2+-phosphate transfection efficiency in low-density neuronal cultures. Nat Protoc. 2006;1(2):695-700. doi: 10.1038/nprot.2006.86. PMID: 17406298.
² Dalby B, Cates S, Harris A, Ohki EC, Tilkins ML, Price PJ, Ciccarone VC. Advanced transfection with Lipofectamine 2000 reagent: primary neurons, siRNA, and high-throughput applications. Methods. 2004 Jun;33(2):95-103. doi: 10.1016/j.ymeth.2003.11.023. PMID: 15121163.
³ Zeitelhofer M, Vessey JP, Xie Y, Tübing F, Thomas S, Kiebler M, Dahm R. High-efficiency transfection of mammalian neurons via nucleofection. Nat Protoc. 2007;2(7):1692-704. doi: 10.1038/nprot.2007.226. PMID: 17641634.
⁴ Charrier C, Joshi K, Coutinho-Budd J, Kim JE, Lambert N, de Marchena J, Jin WL, Vanderhaeghen P, Ghosh A, Sassa T, Polleux F. Inhibition of SRGAP2 function by its human-specific paralogs induces neoteny during spine maturation. Cell. 2012 May 11;149(4):923-35. doi: 10.1016/j.cell.2012.03.034. Epub 2012 May 3. PMID: 22559944; PMCID: PMC3357949.
⁵ Buerli T, Pellegrino C, Baer K, Lardi-Studler B, Chudotvorova I, Fritschy JM, Medina I, Fuhrer C. Efficient transfection of DNA or shRNA vectors into neurons using magnetofection. Nat Protoc. 2007;2(12):3090-101. doi: 10.1038/nprot.2007.445. PMID: 18079708.