BLOG > Publications & Citations > Gene therapy improves outcomes in a mouse model of multiple sulfatase deficiency
Authors: Pham, Vi et al.
Source: Molecular Therapy Volume 32, Issue 11, 3829 - 3846
We are thrilled to share insights from a groundbreaking study titled "Hematopoietic stem cell gene therapy improves outcomes in a clinically relevant mouse model of multiple sulfatase deficiency" published in Molecular Therapy by Pham, Vi et al.
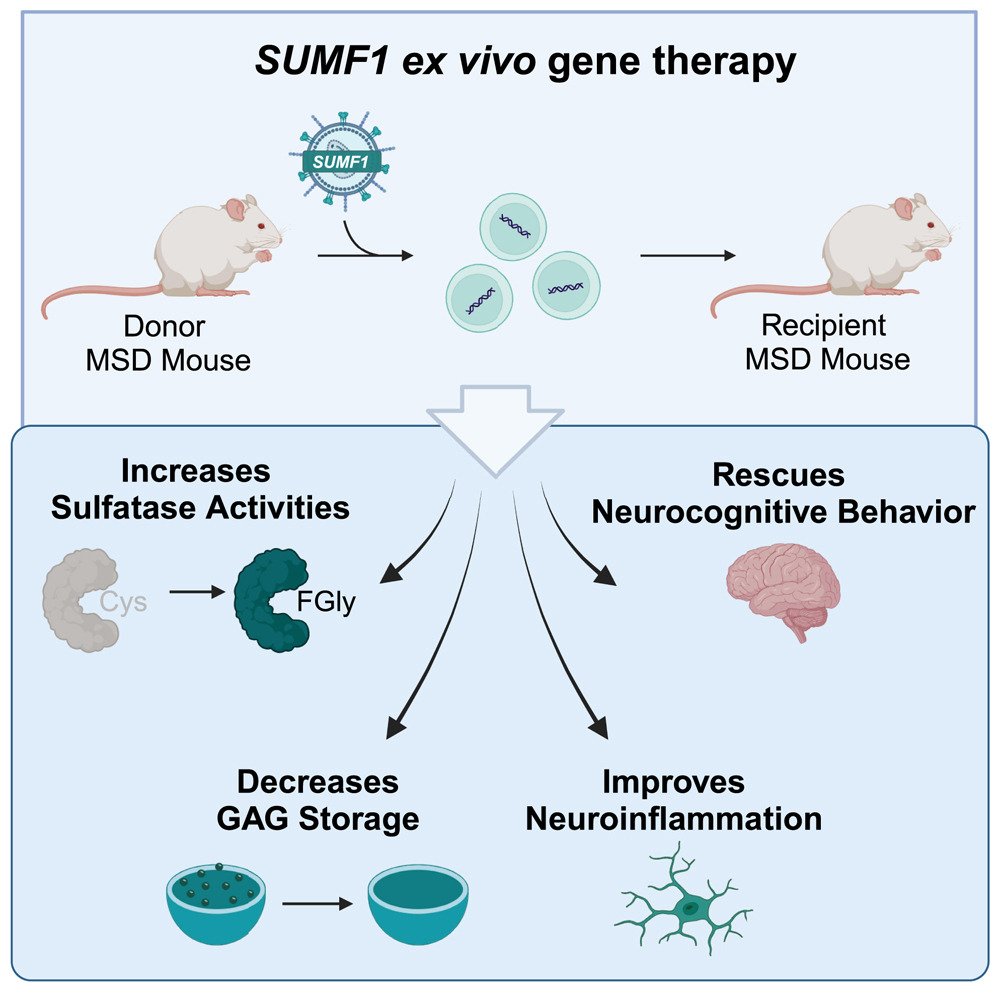
"Multiple sulfatase deficiency (MSD) is a severe, lysosomal storage disorder caused by pathogenic variants in the gene SUMF1, encoding the sulfatase modifying factor formylglycine-generating enzyme. Patients with MSD exhibit functional deficiencies in all cellular sulfatases. The inability of sulfatases to break down their substrates leads to progressive and multi-systemic complications in patients, similar to those seen in single-sulfatase disorders such as metachromatic leukodystrophy and mucopolysaccharidoses IIIA. Here, we aimed to determine if hematopoietic stem cell transplantation with ex vivo SUMF1 lentiviral gene therapy could improve outcomes in a clinically relevant mouse model of MSD. We first tested our approach in MSD patient-derived cells and found that our SUMF1 lentiviral vector improved protein expression, sulfatase activities, and glycosaminoglycan accumulation. In vivo, we found that our gene therapy approach rescued biochemical deficits, including sulfatase activity and glycosaminoglycan accumulation, in affected organs of MSD mice treated post-symptom onset. In addition, treated mice demonstrated improved neuroinflammation and neurocognitive function. Together, these findings suggest that SUMF1 HSCT-GT can improve both biochemical and functional disease markers in the MSD mouse."
Congratulations to all authors for this great article.
Our LentiBlast Premium transduction reagents was used to transduce Lentivirus in Mouse Hematopoietic Stem cells.