BLOG > Publications & Citations > NEW PUBLICATION : Tet-mediated DNA demethylation
Authors: Liyang Ma et al.
Source: Science Advances, 8,eabm3470 (2022)
We are thrilled to share insights from a study entitled "Tet-mediated DNA demethylation regulates specification of hematopoietic stem and progenitor cells during mammalian embryogenesis" published in Science Advances by Liyang Ma et al.
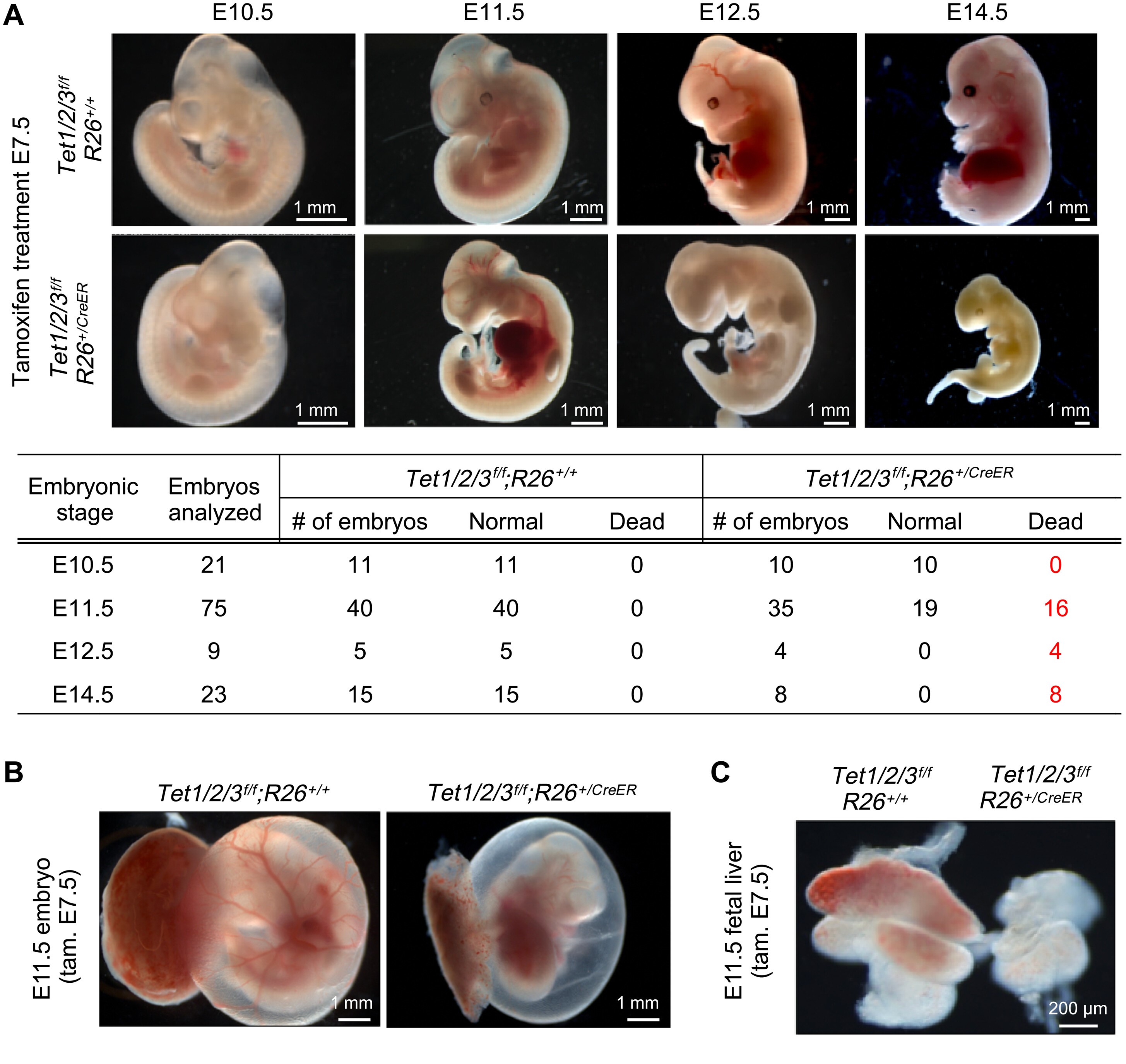
"Ten-eleven translocation (Tet) enzymes promote DNA demethylation by oxidizing 5-methylcytosine. They are expressed during development and are essential for mouse gastrulation. However, their postgastrulation functions are not well established. We find that global or endothelial-specific loss of all three Tet enzymes immediately after gastrulation leads to reduced number of hematopoietic stem and progenitor cells (HSPCs) and lethality in mid-gestation mouse embryos. This is due to defects in specification of HSPCs from endothelial cells (ECs) that compromise primitive and definitive hematopoiesis. Mechanistically, loss of Tet enzymes in ECs led to hypermethylation and down-regulation of NFκB1 and master hematopoietic transcription factors (Gata1/2, Runx1, and Gfi1b). Restoring Tet catalytic activity or overexpression of these factors in Tet-deficient ECs rescued hematopoiesis defects. This establishes Tet enzymes as activators of hematopoiesis programs in ECs for specification of HSPCs during embryogenesis, which is distinct from their roles in adult hematopoiesis, with implications in deriving HSPCs from pluripotent cells."
Congratulations to all authors for this great article.
Our LentiBlast Premium enhancer was used to transduce lentivirus into explant cultures of mice aortagonad-mesonephros (AGM).
Read the article See our LentiBlast Premium