BLOG > mRNA & LNP > Lipid Nanoparticles (LNPs): A complete guide for researchers
Summary:
1. What are lipid nanoparticles?
2. How lipid nanoparticles are produced?
3. Experimental results on NanOZ-LNPs performance
Lipid nanoparticles (LNPs) have become essential tools in nucleic acid delivery, gene therapy, and mRNA vaccine development. This guide explains what LNPs are, how they are produced, and presents experimental results on their performance in research.
1. What are lipid nanoparticles?
LNPs are liposome-like structures, specially geared towards encapsulating a broad variety of nucleic acids (both, mRNA, miRNA, siRNA, gRNA, lncRNA, circRNA and DNA) or active pharmaceutical ingredients (APIs, e.g. small molecules, proteins); a LNP consists in an aqueous core surrounded by a lipidic shell based on a combination of different compounds, each having its own role (Figure 1). Generally, LNPs are composed of four families of chemicals: a complexing aminated lipid (e.g. ionizable cationic), an helper phospholipid (DSPC, DOPC or DOPE), cholesterol and a pegylated (PEG) lipid at defined ratio to potentiate the nucleic acid/APIs activity.
- Complexing lipids are usually comprising a ionizable/positively-charged head group, an hydrocarbon chain or cholesterol derivate, attached via a linker (e.g. glycerol). The charged head of the lipid would interact by electrostatic bindings with anionic nucleic acids to allow their entrapment into LNPs during their formulation. In addition, these lipids can mediate interactions between LNPs and the cellular plasma, and facilitate cell uptake and endosomal release of the cargo. New series of ionizable lipids have highly been developed to potentiate RNA delivery in vivo. Ionizable lipids expose a negative-neutral surface charge under physiological pH (7.4), thereby preventing serum protein opsonization. The pH in the endosome goes acidic, below the pKa of the lipid, inducing protonation and thus the LNPs destabilisation associated with the nucleic acid release.
- Helper lipids such as phospholipids favour colloidal stability and gene delivery efficiency meanwhile increasing systemic circulation of the NPs. Phosphatidylcholine lipids (PCs) compose cell membranes. Saturated PCs, such as 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), are the most representative helper lipid used to form highly stable LNPs due to its high melting temperature (Tm).
- Cholesterol is commonly used in liposomes and LNPs. It helps stabilizing the lipidic shell and promotes membrane fusion.
- PEGylated lipid into LNPs increases their colloidal stability, resistance to opsonization and reticuloendothelial clearance. Pegylation of the nanoparticles results in long-term biodistribution, better tumour penetration and accumulation by leading to neutral surface charge and by reducing the size of LNPs.
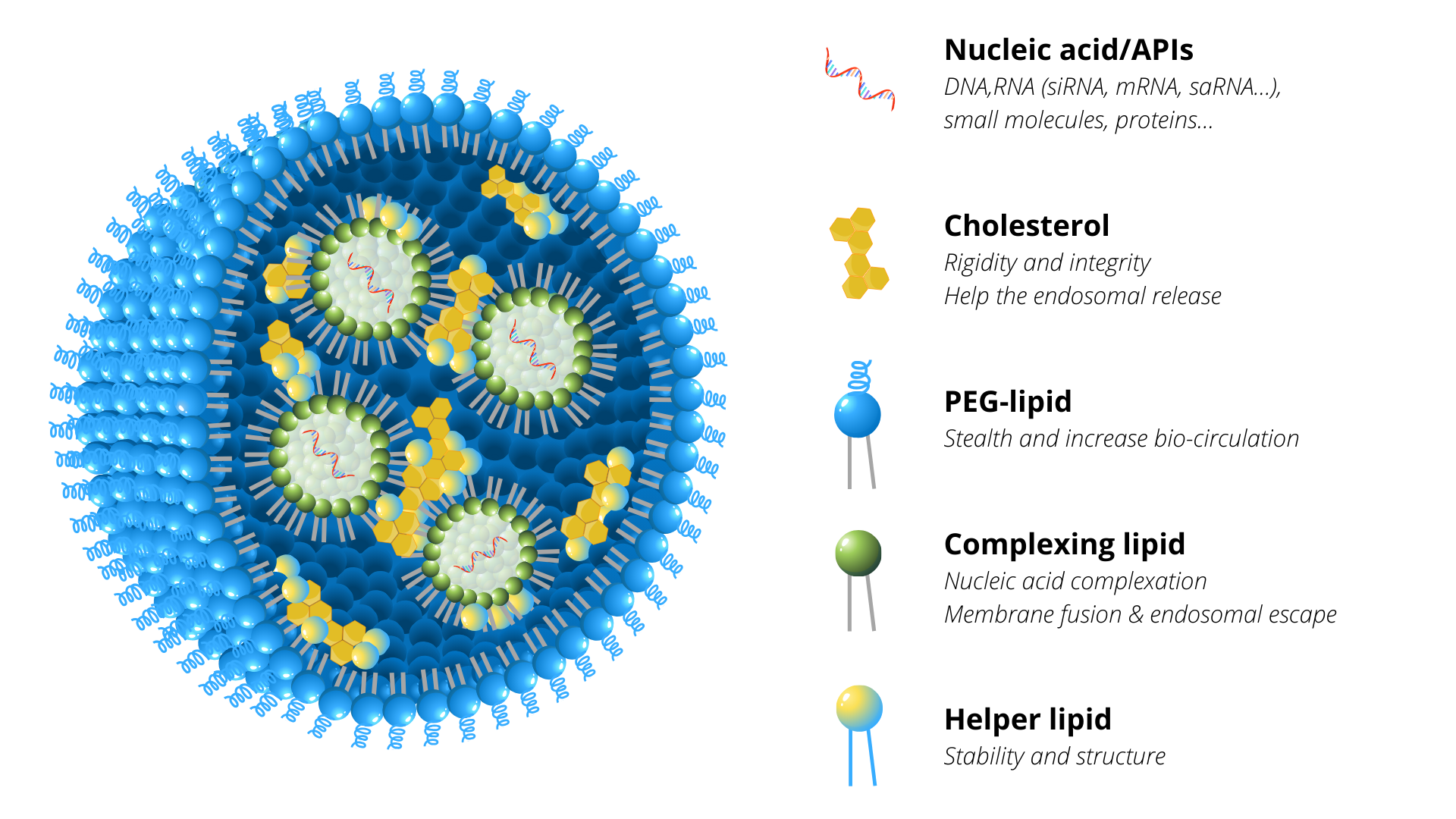
Figure 1: Schematic representation of lipid nanoparticles (LNPs) composed by a mixture of four chemical compounds: complexing lipid, helper phospholipid, cholesterol and stealth-lipid each with their own specific function and at defined ratio to potentiate nucleic acid activity.
2. How lipid nanoparticles are produced?
Different synthetic methods for RNA-loaded LNPs have been adopted to generate efficient drug carriers. Classical preparation involve a traditional rehydration of a lipidic thin-film with an aqueous buffer containing the RNA drugs. The major drawback to this method is the random nature of the complexes, reproducibility and the polydispersity of the resulting nanoparticles, requiring the use of an additional extrusion.
Accordingly, the development of microfluidic techniques in the early 2000s allows reproducible, relevant and scalable LNP production. Therefore, NanOZ-LNPs are mainly formed throughout our microfluidics technology platform. The microfluidics system, based on a pressure-driven flow control, provides tools to manipulate liquids, droplets, cell and particles within micro-channel geometries. To form well-structured lipid nanosystems, typically a stream of lipid in alcohol solution is flown by fast mixing against an anti-solvent containing active ingredients (e.g. RNA in an acidic aqueous phase) at the junction of the microfluidic chip (Figure 2).
In the last 20 years, OZ Biosciences has developed strong expertise in aminated lipids, which allowed the screening of several dozens formulations in order to develop optimized OZB LNPs referred as NanOZ-LNPs.
NanOZ-LNPs have been designed as safe and advanced nanomaterials to potentiate nucleic acids/APIs activity through their effective encapsulation and delivery of payload to specific cell types and tissues.
Check NanOZ-LNPsThe API-loaded LNPs self-assemble at the intersection of the chip by nucleation-growth process before being collected at the output of the system. The processing has the advantage to directly encapsulate the molecule of interest into the NP and to quickly form LNPs in various volume range, from hundred µL to several tens of mL. Once collected, alcohol solvent and free molecules are removed from LNPs by dialysis or tangential flow filtration (TFF).
These processes stabilize the LNP structure and adjust the pH to neutral, which is required for further in vitro and in vivo applications. LNPs can be sterilized by filtration and are generally stored at -80°C with the addition of cryoprotectant.
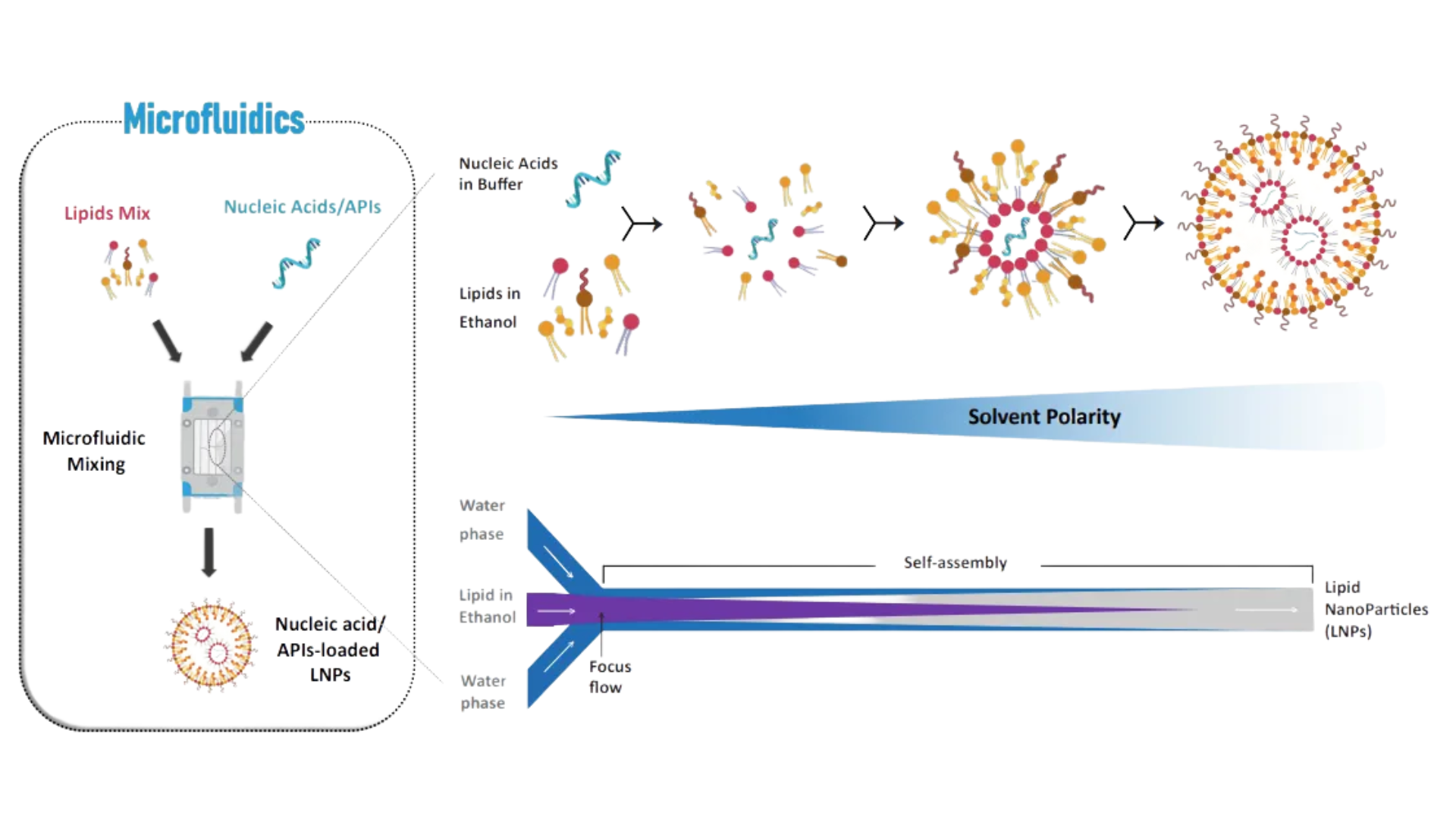
Figure 2: LNPs manufacturing with precise control and high reproducibility based on fastmixing microfluidics technolog.
Key points:
- LNPs are formed through nucleation/growth process.
- The particule size can be tuned by modulating TFR and FRR.
- API can be brought by continous (aqueous) phase or inner (lipophilic) phase.
Microfluidics brings the possibility to work using a wide range of nucleic acids (e.g. mRNA, siRNA, saRNA, DNA) and to rapidly screen a large library of lipids in order to optimize the formulation efficacy for gene delivery. Once the LNP formulation optimized, microfluidic technology provides the huge benefit to easily reproduce and scale-up the manufacturing of the delivery systems. Given all these aspects, microfluidics technology offers unprecedented precision and control over the production of nanoparticles.
3. Experimental results on NanOZ-LNPs performance
Our resulting NanOZ-LNPs are characterized by different analytical techniques including dynamic light scattering (DLS) to monitor their hydrodynamic size, polydispersity index (PdI) and zeta potential and electronic microscopies (cryoTEM, negative stain EM) to evaluate their morphologies (Figure 3). The nucleic acid entrapment rate is determined by DNA- or RNA-quantitation kit assay and proved to be very high (typically ≥90%).
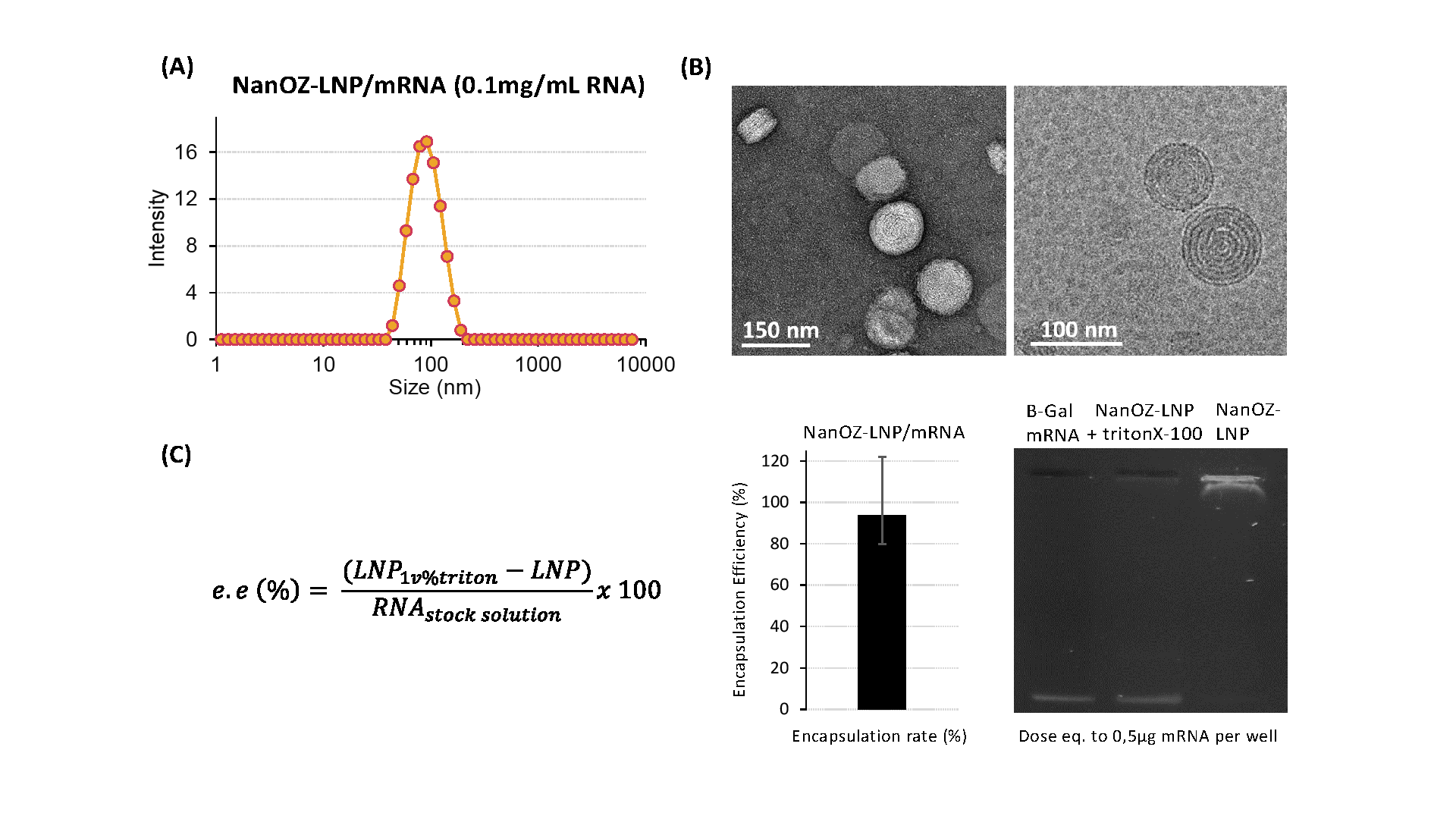
Figure 3: (A) Dynamic Light Scattering (DLS), and (B) negative stain and Cryo-TEM micrographs of around 85nm mRNA-loaded LNPs. (C) Encapsulation efficiency (e.e.) of NanOZ-LNP encapsulated β-Gal mRNA determined by SybrGold® and gel electrophoresis (at 0.5µg mRNA dose). Samples that have or not been destabilized by surfactant (± 1v% TritonX-100) reveal the integrity of the mRNA payload.
To learn more about lipid nanoparticles (LNPs), take a look at our other ressources and discover our products: